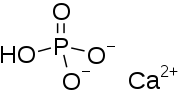
Dicalcium phosphate
(H2O)2_from_JCS_A_Curry%2C_N.A.%3B_Jones%2C_D.W._(1971.jpg) | |
 | |
| Names | |
|---|---|
| IUPAC name
Calcium hydrogen phosphate dihydrate | |
| Other names
Calcium Hydrogen Phosphate Phosphoric acid, calcium salt (1:1) | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.028.933 |
PubChem CID |
|
| UNII | |
| |
| |
| Properties | |
| CaHPO4 | |
| Molar mass | 136.06 g/mol (anhydrous) 172.09 (dihydrate) |
| Appearance | white powder |
| Odor | odorless |
| Density | 2.929 g/cm3 (anhydrous) 2.31 g/cm3 (dihydrate) |
| Melting point | decomposes |
| 0.02 g/100 mL (anhydrous) 0.02 g/100 mL (dihydrate) | |
| Structure | |
| triclinic | |
| Hazards | |
| NFPA 704 | |
| Flash point | Non-flammable |
| Related compounds | |
Other anions |
Calcium pyrophosphate |
Other cations |
Magnesium phosphate Monocalcium phosphate Tricalcium phosphate Strontium phosphate |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Dicalcium phosphate is the calcium phosphate with the formula CaHPO4 and its dihydrate. The "di" prefix in the common name arises because the formation of the HPO42– anion involves the removal of two protons from phosphoric acid, H3PO4. It is also known as dibasic calcium phosphate or calcium monohydrogen phosphate. Dicalcium phosphate is used as a food additive, it is found in some toothpastes as a polishing agent and is a biomaterial.[1][2]
Preparation
Dibasic calcium phosphate is produced by the neutralization of calcium hydroxide with phosphoric acid, which precipitates the dihydrate as a solid. At 60 °C the anhydrous form is precipitated:[3]
- H3PO4 + Ca(OH)2 → CaHPO4
To prevent degradation that would form hydroxyapatite, sodium pyrophosphate or trimagnesium phosphate octahydrate are added when for example, dibasic calcium phosphate dihydrate is to be used as a polishing agent in toothpaste.[1]
In a continuous process CaCl2 can be treated with (NH4)2HPO4 to form the dihydrate:
- CaCl2 + (NH4)2HPO4 → CaHPO4•2H2O
A slurry of the dihydrate is then heated to around 65–70 °C to form anhydrous CaHPO4 as a crystalline precipitate, typically as flat diamondoid crystals, which are suitable for further processing.[4]
Dibasic calcium phosphate dihydrate is formed in "brushite" calcium phosphate cements (CPC's), which have medical applications. An example of the overall setting reaction in the formation of "β-TCP/MCPM" (β-tricalcium phosphate/monocalcium phosphate) calcium phosphate cements is:[5]
- Ca3(PO4)2 + Ca(H2PO4)2•H2O + 7 H2O → 4 CaHPO4•2H2O
Structure
Three (3) forms of dicalcium phosphate are known:
- dihydrate, CaHPO4•2H2O ('DPCD'), the mineral brushite
- hemihydrate, CaHPO4•0.5H2O
- anhydrous CaHPO4, ('DCPA'), the mineral monetite. Below pH 4.8 the dihydrate and anhydrous forms of dicalcium phosphate are the most stable (insoluble) of the calcium phosphates.
The structure of the anhydrous and dihydrated forms have been determined by X-ray crystallography. The dihydrate (shown in table above) adopts a layered structure.[6]
2portion.jpg)
Uses and occurrence
Dibasic calcium phosphate is mainly used as a dietary supplement in prepared breakfast cereals, dog treats, enriched flour, and noodle products. It is also used as a tableting agent in some pharmaceutical preparations, including some products meant to eliminate body odor. Dibasic calcium phosphate is also found in some dietary calcium supplements (e.g. Bonexcin). It is used in poultry feed. It is also used in some toothpastes as a tartar control agent.[7]
Heating dicalcium phosphate gives dicalcium diphosphate, a useful polishing agent:
- 2 CaHPO4 → Ca2P2O7 + H2O
In the dihydrate (brushite) form it is found in some kidney stones and in dental calculi.[8][3]
References
- 1 2 Corbridge, D. (1995). "Chapter 3: Phosphates". Studies in inorganic Chemistry vol. 20. Elsevier Science B.V. pp. 169–305. ISBN 0-444-89307-5. Retrieved January 30, 2015. – via ScienceDirect (Subscription may be required or content may be available in libraries.)
- ↑ Salinas, Antonio J.; Vallet-Regi, Maria (2013). "Bioactive ceramics: from bone grafts to tissue engineering". RSC Advances. Royal Society of Chemistry. 3 (28): 11116–11131. doi:10.1039/C3RA00166K. Retrieved 15 February 2015. (Subscription required (help)).
- 1 2 Rey, C.; Combes, C.; Drouet, C.; Grossin, D. (2011). "1.111 - Bioactive Ceramics: Physical Chemistry". In Ducheyne, Paul. Comprehensive Biomaterials. 1. Elsevier. pp. 187–281. doi:10.1016/B978-0-08-055294-1.00178-1. ISBN 978-0-08-055294-1. – via ScienceDirect (Subscription may be required or content may be available in libraries.)
- ↑ Ropp, R.C. (2013). "Chapter 4 - Group 15 (N, P, As, Sb and Bi) Alkaline Earth Compounds". Encyclopedia of the Alkaline Earth Compounds. 1. Elsevier. doi:10.1016/B978-0-444-59550-8.00004-1. ISBN 978-0-444-59550-8. – via ScienceDirect (Subscription may be required or content may be available in libraries.)
- ↑ Tamimi, Faleh; Sheikh, Zeeshan; Barralet, Jake (February 2012). "Dicalcium phosphate cements: Brushite and monetite". Acta Biomaterialia. 8 (2): 474–484. doi:10.1016/j.actbio.2011.08.005. ISSN 1742-7061. – via ScienceDirect (Subscription may be required or content may be available in libraries.)
- ↑ Curry, N.A.; Jones, D.W. (1971). "Crystal structure of brushite, calcium hydrogen orthophosphate dihydrate: A neutron-diffraction investigation". of the Chemical Society A: 3725–3729.
- ↑ Klaus Schrödter, Gerhard Bettermann, Thomas Staffel, Friedrich Wahl, Thomas Klein, Thomas Hofmann "Phosphoric Acid and Phosphates" in Ullmann’s Encyclopedia of Industrial Chemistry 2008, Wiley-VCH, Weinheim. doi:10.1002/14356007.a19_465.pub3
- ↑ Pak, Charles Y.C, Poindexter, John R, Adams-Huet, Beverley, Pearle, Margaret S (July 2003). "Predictive value of kidney stone composition in the detection of metabolic abnormalities". The American Journal of Medicine. 115 (1): 26–32. doi:10.1016/S0002-9343(03)00201-8. ISSN 0002-9343. – via ScienceDirect (Subscription may be required or content may be available in libraries.)
