2-Ethylhexanol
 | |
 | |
| Names | |
|---|---|
| IUPAC name
2-Ethylhexan-1-ol[1] | |
| Identifiers | |
3D model (JSmol) |
|
| 1719280 | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.002.941 |
| EC Number | 203-234-3 |
| KEGG | |
| MeSH | 2-ethylhexanol |
PubChem CID |
|
| UNII | |
| |
| |
| Properties | |
| C8H18O | |
| Molar mass | 130.23 g·mol−1 |
| Appearance | Colourless liquid |
| Density | 833 mg mL−1 |
| Melting point | −76 °C (−105 °F; 197 K) |
| Boiling point | 180 to 186 °C; 356 to 367 °F; 453 to 459 K |
| log P | 2.721 |
| Vapor pressure | 30 Pa (at 20 °C) |
Refractive index (nD) |
1.431 |
| Thermochemistry | |
Heat capacity (C) |
317.5J K−1 mol−1 |
Std molar entropy (S |
347.0 J K−1 mol−1 |
Std enthalpy of formation (ΔfH |
−433.67–−432.09 kJ mol−1 |
Std enthalpy of combustion (ΔcH |
−5.28857–−5.28699 MJ mol−1 |
| Hazards | |
| GHS pictograms | 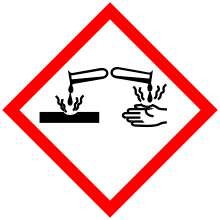  |
| GHS signal word | DANGER |
| H312, H315, H318, H335 | |
| P261, P280, P305+351+338 | |
| Flash point | 81 °C (178 °F; 354 K) |
| 290 °C (554 °F; 563 K) | |
| Explosive limits | 0.88–9.7% |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose) |
|
| US health exposure limits (NIOSH): | |
PEL (Permissible) |
none[2] |
REL (Recommended) |
TWA 50 ppm (270 mg/m3) [skin][2] |
IDLH (Immediate danger) |
N.D.[2] |
| Related compounds | |
Related alkanol |
Propylheptyl alcohol |
Related compounds |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
2-Ethylhexanol (abbreviated 2-EH) is a branched, eight-carbon chiral alcohol. It is a colorless liquid that is poorly soluble in water but soluble in most organic solvents. It is produced on a massive scale (>2,000,000,000 kg/y) for use in numerous applications such as solvents, flavors, and fragrances and especially as a precursor for production of other chemicals such as emollients and plasticizers.[3] It is encountered in natural plant fragrances, and the odor has been reported as "heavy, earthy, and slightly floral" for the R enantiomer and "a light, sweet floral fragrance" for the S enantiomer.[4]
Properties and applications
The branching in 2-ethylhexanol inhibits its crystallization due to packing disruption; this results in a very low freezing point. Esters of 2-ethylhexanol are similarly affected and it therefore finds application as a feedstock in the production of plasticizers and lubricants, where its presence helps reduce viscosity and lower freezing points.
Almost all 2-ethylhexanol manufactured is used as a precursor for the synthesis of the diester bis(2-ethylhexyl) phthalate (DEHP), a plasticizer. Because it is a fatty alcohol, its esters tend to have emollient properties.
It is also commonly used as a low volatility solvent. 2-Ethylhexanol can also be used as an octane booster when reacted with nitric acid.
Industrial production
2-Ethylhexanol is produced industrially by the aldol condensation of n-butyraldehyde, followed by hydrogenation of the resulting hydroxyaldehyde. About 2,500,000 tons are prepared in this way annually.[5][6]
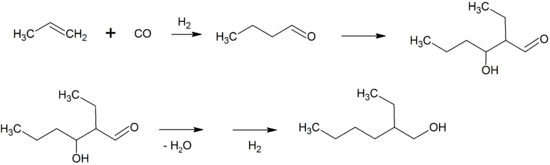
The n-butyraldehyde is made by hydroformylation of propylene, either in a self-contained plant or as the first step in a fully integrated facility. Most facilities make n-butanol and isobutanol in addition to 2-ethylhexanol. The overall process is very similar to that of the Guerbet reaction, by which it may also be produced.[7]
Health effects
2-Ethylhexanol exhibits low toxicity in animal models, with LD50 ranging from 2-3 g/kg (rat).[3]
Nomenclature
Although isooctanol (and the derived isooctyl prefix) is commonly used in industry to refer to 2-ethylhexanol and its derivatives, IUPAC naming conventions[8] dictate that this name is properly applied to another isomer of octanol, 6-methylheptan-1-ol. The Chemical Abstracts Service likewise indexes isooctanol (CAS# 26952-21-6) as 6-methylheptan-1-ol.
See also
References
- ↑ "2-ethylhexanol - Compound Summary". PubChem Compound. USA: National Center for Biotechnology Information. 16 September 2005. Identification and Related Records. Retrieved 29 January 2012.
- 1 2 3 "NIOSH Pocket Guide to Chemical Hazards #0354". National Institute for Occupational Safety and Health (NIOSH).
- 1 2 Helmut Bahrmann, Heinz-Dieter Hahn, Dieter Mayer (2005). "2-Ethylhexanol". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a10_137.
- ↑ Klaus Rettinger; Christian Burschka; Peter Scheeben; Heike Fuchs; Armin Mosandl (1991). "Chiral 2-alkylbranched acids, esters and alcohols. Preparation and stereospecific flavour evaluation". Tetrahedron: Asymmetry. 2 (10): 965–968. doi:10.1016/S0957-4166(00)86137-6.
- ↑ C. Kohlpaintner, M. Schulte, J. Falbe, P. Lappe, J. Weber (2008). "Aldehydes, Aliphatic". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a01_321.pub2.
- ↑ Ashford’s Dictionary of Industrial Chemicals, Third edition, 2011, page 4180-4181.
- ↑ Miller, Robert; Bennett, George (January 1961). "Producing 2-Ethylhexanol by the Guerbet Reaction". Industrial & Engineering Chemistry. 53 (1): 33–36. doi:10.1021/ie50613a027.
- ↑ IUPAC Blue Book, A2.25