Cyclobutadiene
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
Cyclobuta-1,3-diene | |||
| Other names
1,3-Cyclobutadiene Cyclobutadiene [4]Annulene | |||
| Identifiers | |||
3D model (JSmol) |
|||
| ChEBI | |||
| ChemSpider | |||
PubChem CID |
|||
| |||
| |||
| Properties | |||
| C4H4 | |||
| Molar mass | 52.07 g/mol | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
Cyclobutadiene is an organic compound with the formula C4H4. It is very reactive owing to its tendency to dimerize. Although the parent compound has not been isolated, some substituted derivatives are robust and a single molecule of cyclobutadiene is quite stable. Since the compound degrades by a bimolecular process, the species can be observed by matrix isolation techniques at temperatures below 35 K. It is thought to adopt a rectangular structure.[1][2]
Structure and reactivity
The compound is the prototypical antiaromatic hydrocarbon with 4 π-electrons. It is the smallest [n]-annulene ([4]-annulene). Its rectangular structure is the result of the Jahn–Teller effect, which distorts the molecule, converting the triplet to a singlet ground state.[3] The electronic states of cyclobutadiene have been explored with a variety of computational methods.[4] The rectangular structure is consistent with the existence of two different 1,2-dideutero-1,3-cyclobutadiene stereoisomers. This distortion indicates that the pi electrons are localized, in agreement with Hückel's rule which predicts that a π-system of 4 electrons is not aromatic.
In principle, another situation is possible. Namely, cyclobutadiene could assume an undistorted square geometry, if it adopts a triplet spin state. While a theoretical possibility, the triplet form of the parent cyclobutadiene and its substituted derivatives remained elusive for decades. However, in 2017, the square triplet excited state of 1,2,3,4-tetrakis(trimethylsilyl)-1,3-cyclobutadiene was observed spectroscopically, and a singlet-triplet gap of EST = 13.9 kcal/mol was measured for this compound.[5]
Synthesis
Several cyclobutadiene derivatives have been isolated with steric bulky substituents. Orange tetrakis(tert-butyl)cyclobutadiene arises by thermolysis of its isomer tetra-tert-butyltetrahedrane. Although the cyclobutadiene derivative is stable (with respect to dimerization), it decomposes upon contact with O2.[6][7]
Trapping
Samples of cyclobutadiene are unstable since the compound dimerizes at temperatures above 35 K by a Diels-Alder reaction.[8] By suppressing bimolecular decomposition pathways, cyclobutadiene is well-behaved. Thus it has been generated in an hemicarceplex.[2] The inclusion compound is generated by photodecarboxylation of bicyclopyran-2-one.[9] When released from the host-guest complex, cyclobutadiene dimerizes and then converts to cyclooctatetraene.
After numerous attempts, cyclobutadiene was first generated by oxidative degradation of cyclobutadieneiron tricarbonyl with ammonium cerium(IV) nitrate.[10][11] When liberated from the iron complex, cyclobutadiene reacts with electron-deficient alkynes to form a Dewar benzene:[12]
The Dewar benzene converts to dimethyl phthalate on heating at 90 °C.
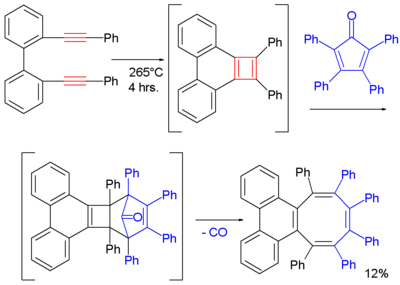
One cyclobutadiene derivative is also accessible through a [2+2]cycloaddition of a di-alkyne. In this particular reaction the trapping reagent is 2,3,4,5-tetraphenylcyclopenta-2,4-dienone and one of the final products (after expulsion of carbon monoxide) is a cyclooctatetraene:[13]
See also
References
- ↑ "A theoretical study of the structure of cyclobutadiene H. Kollmar, V. Staemmler; J. Am. Chem. Soc". 99. 1977: 3583–3587. doi:10.1021/ja00453a009.
- 1 2 "The Taming of Cyclobutadiene Donald J. Cram, Martin E. Tanner, Robert Thomas". Angewandte Chemie International Edition in English. 30: 1024–1027. 1991. doi:10.1002/anie.199110241.
- ↑ Peter Senn (1992). "A Simple Quantum Mechanical Model that Illustrates the Jahn-Teller Effect". J. Chem. Educ. 69: 819. doi:10.1021/ed069p819.
- ↑ Balkova, A.; Bartlett, R. J. J. Chem. Phys. 1994, 101, 8972–8987.
- ↑ Kostenko, Arseni; Tumanskii, Boris; Kobayashi, Yuzuru; Nakamoto, Masaaki; Sekiguchi, Akira; Apeloig, Yitzhak (2017-07-03). "Spectroscopic Observation of the Triplet Diradical State of a Cyclobutadiene". Angewandte Chemie International Edition. 56 (34): 10183–10187. doi:10.1002/anie.201705228. ISSN 1433-7851.
- ↑ Günther Maier, Stephan Pfriem, Ulrich Schäfer, Rudolf Matusch (1978). "Tetra-tert-butyltetrahedrane". Angew. Chem. Int. Ed. Engl. 17: 520. doi:10.1002/anie.197805201.
- ↑ Hermann Irngartinger, Norbert Riegler, Klaus-Dieter Malsch, Klaus-Albert Schneider, Günther Maier (1980). "Structure of Tetra-tert-butylcyclobutadiene". Angewandte Chemie International Edition in English. 19: 211–212. doi:10.1002/anie.198002111.
- ↑ Carey, Francis A.; Sundberg, Richard J. (2007). Advanced Organic Chemistry: Part A: Structure and Mechanisms (5th ed.). Springer. p. 725. ISBN 978-0-387-44897-8.
- ↑ E. J. Corey, Jacques Streith (1964). "Internal Photoaddtion Reactions of 2-Pyrone and N-Methyl-2-pyridone: A New Synthetic Approach to Cyclobutadiene". J. Am. Chem. Soc. 86: 950–951. doi:10.1021/ja01059a059.
- ↑ G. F. Emerson, L. Watts, R. Pettit (1965). "Cyclobutadiene- and Benzocyclobutadiene-Iron Tricarbonyl Complexes". J. Am. Chem. Soc. 87: 131–133. doi:10.1021/ja01079a032.
- ↑ R. Pettit, J. Henery (1970). "Cyclobutadieneiron tricarbonyl". Organic Syntheses. 50: 21. doi:10.15227/orgsyn.050.0021.
- ↑ L. Watts, J. D. Fitzpatrick, R. Pettit (1965). "Cyclobutadiene". J. Am. Chem. Soc. 87: 3253–3254. doi:10.1021/ja01092a049.
- ↑ Chung-Chieh Lee, Man-kit Leung, Gene-Hsiang Lee, Yi-Hung Liu, Shie-Ming Peng (2006). "Revisit of the Dessy-White Intramolecular Acetylene-Acetylene [2 + 2] Cycloadditions". J. Org. Chem. 71: 8417–8423. doi:10.1021/jo061334v.