Chain-growth polymerization
proceeds exclusively by reaction(s) between monomer(s) and active site(s)
on the polymer chain with regeneration of the active site(s) at the end of
each growth step.[1]
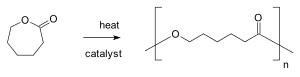
Chain-growth polymerization or chain polymerization (IUPAC recommended term) is a polymerization mechanism in which monomer molecules add onto the active site of a growing polymer chain one at a time.[2] Growth of the polymer occurs only the active sites on the chain, which are typically at the chain-end(s). Addition of each monomer unit regenerates the active site.[3] In chain growth polymerization, an activated species (initiator or active center) adds one monomer molecule to create a new active center (propagation step), which again adds another monomer molecule to create another active center and so on, so that the chain growth proceeds as a chemical chain reaction.
Polyethylene, polypropylene, and polyvinyl chloride (PVC) are common types of plastics made by chain polymerization. They are the primary component of four of the plastics specifically labeled with recycling codes and are used extensively in packaging.
Mechanism
Chain-growth polymerization can be understood with the chemical equation:
where n is the degree of polymerization and M is some form of monomer such as an unsaturated compound: an alkene (vinyl polymers) or an alicyclic compound (ring-opening polymerization).
Chain polymerization can also consist of sequential condensation reactions, when the process is known as condensative chain polymerization. Examples of condensative chain polymerization are catalyst transfer polymerization and many enzyme-catalyzed polymerizations such as biological formation of peptides, RNA and DNA.
This type of polymerization results in high molecular weight polymer being formed at low conversion. This final weight is determined by the rate of propagation compared to the rate of individual chain termination, which includes both chain transfer and chain termination steps. Above a certain ceiling temperature, no polymerization occurs.
Steps
Chain-growth polymerization usually has the following steps:
- chain initiation, usually by means of an initiator which generates active centers and starts the chemical process. Typical initiators include organic compounds with a labile group: e.g. azo (-N=N-), disulfide (-S-S-), or peroxide (-O-O-). Two examples are benzoyl peroxide and AIBN.
- chain propagation by an active center on the growing polymer molecule, which adds one monomer molecule to form a new polymer molecule which is one repeat unit longer with a new active center.
- chain transfer terminates the chain, but the active site is transferred to a new chain. This can occur by reaction with the solvent, monomer, or another polymer molecule. Reaction with another polymer molecule results in the formation of a branched polymer.
- chain termination, which occurs either by combination or disproportionation. Termination, in radical polymerization, is when the free radicals combine and is the end of the polymerization process.
The active center can be one of a number of different types:
- free radical in radical polymerization, for example, polystyrene, sometimes seen as packing peanuts, is produced by polymerizing styrene with Benzoyl peroxide as its radical initiator
- carbocation in cationic polymerization, an example is Isobutyl synthetic rubber, initiated by Aluminium chloride ionizing isobutylene
- carbanion in anionic polymerization
- organometallic complex in coordination polymerization.
Under the necessary reaction conditions, an addition polymerization can be considered a living polymerization. This is most often seen with anionic polymerization as it can be easy to perform without termination steps.
Comparison with other polymerization methods
The distinction between step-growth polymerization and chain-growth polymerization was introduced by Paul Flory in 1953.[4]
IUPAC deprecates the term step-growth polymerization and recommends use of the terms polyaddition, when the propagation steps are addition reactions and no molecules are evolved during these steps, and polycondensation when the propagation steps are condensation reactions and molecules are evolved during these steps.
Chain polymerization and polyaddition are two different concepts. Polyaddition, unlike chain polymerization, involves addition reactions between species of all degrees of polymerization. Polyurethane formation is an example of polyaddition.
The distinction between polyaddition and polycondensation was introduced by Wallace Hume Carothers in 1929.[5][6]
References
- ↑ Penczek, Stanisław; Moad, Graeme (2008). "Glossary of terms related to kinetics, thermodynamics, and mechanisms of polymerization (IUPAC Recommendations 2008)" (PDF). Pure and Applied Chemistry. 80 (10): 2163–2193. doi:10.1351/pac200880102163.
- ↑ Introduction to Polymers 1987 R.J. Young Chapman & Hall ISBN 0-412-22170-5
- ↑ Jenkins, A. D.; Kratochvíl, P.; Stepto, R. F. T.; Suter, U. W. (1996). "Glossary of basic terms in polymer science (IUPAC Recommendations 1996. See definition 3.6)" (PDF). Pure and Applied Chemistry. 68 (12): 2287–2311. doi:10.1351/pac199668122287.
- ↑ Susan E. M. Selke, John D. Culter, Ruben J. Hernandez, "Plastics packaging: Properties, processing, applications, and regulations", Hanser, 2004, p.29. ISBN 1-56990-372-7
- ↑ W. H. Carothers (1929). "Studies On Polymerization And Ring Formation. I. An Introduction To The General Theory Of Condensation Polymers". Journal of the American Chemical Society. 51 (8): 2548–59. doi:10.1021/ja01383a041.
- ↑ Paul J. Flory, "Principles of Polymer Chemistry", Cornell University Press, 1953, p.39. ISBN 0-8014-0134-8