Catalyst transfer polymerization
Catalyst transfer polymerization (CTP), or Catalyst Transfer Polycondensation, is a type of living chain-growth polymerization that is used for synthesizing conjugated polymers[1]. Benefits to using CTP over other methods are lower polydispersity and greater control over number average molecular weight in the resulting polymer sample, but very few monomers have been demonstrated to undergo CTP. [2]
History
The first reports of CTP came simultaneously from the labs of Yokozawa[3] and McCullough[4] in 2004, with the recognition that polythiophene can be synthesized with low dispersity and control over molecular weight when a grignard thiophene monomer was polymerized with Nickel(1,2-bis(diphenylphosphino)ethane)dichloride. This recognition sparked interest in the system, and its expansion to other monomers. Despite major advances in the field, relatively few polymers can be synthesized via CTP because the lack of a "universal CTP catalyst" means that every monomer must be paired with a different catalyst in order to obtain ideal living chain-growth behavior.[5] This need to pair monomers to catalysts means most conjugated polymers are synthesized via palladium catalyzed step-growth polycondensation cross-coupling reactions.
Characteristics
CTP is exclusively performed on arene monomers to give conjugated polymers. The polymers obtained from a true CTP are often low dispersity and contain consistent end groups (two on each chain, one put on the polymer chain end during initiation, and the other put on during termination). These result from the fact that CTP is a living, chain growth polymerization, and mean that the average molecular weight of the polymers from a CTP reaction can be tuned by changing the relative amounts of catalyst and monomer at the start of the reaction.
CTP is thought to be important in that control over molecular weight (imparted by CTP) means control over conjugation length in conjugated polymers. Average conjugation length of a conjugated polymer can have a large impact in applications such as solar cells and transistors, both fields that are starting to use organic electronics.
Types
CTP is closely related to many group 10 metal catalyzed cross coupling reactions (see Mechanism below), and often, monomers used in CTP contain magnesium-, zinc-, boron-, and tin-based transmetallating groups, giving rise to Kumada CTP (K-CTP), Negishi CTP (N-CTP), Suzuki CTP (B-CTP), and Stille CTP (S-CTP) reactions, respectively.
Mechanism
The mechanism of CTP has been debated. The living chain-growth nature of CTP can be explained by the existence of a π-complex (as described in this section) but can also be explained via polymer reactivity. A common feature of both pathways is their similarity to other nickel and palladium catalyzed cross-coupling reactions.
Initiation

Initiation from a nickel(II) species involves two monomers transmetalating onto the nickel center to form a complex that can easily undergo reductive elimination. The complex formed after reductive elimination is characterized by the metal, now nickel(0), bound to the π system of the monomer. This π-complex means that the catalysts is not free to dissociate and start polymer chains, forcing monomers to add to the growing chain. The catalyst migrates, via ring-walking, to the π-bond adjacent to the C-X bond at the end of the dimer, allowing oxidative addition to occur, forming the active polymer-nickel(II)-bromide catalyst.[6]
Propagation

The propagation steps of CTP occurs through a similar cycle of transmetalation, reductive elimination, ring walking, and oxidative addition. The existence of a π-complex allows for the polymerization to be controlled as it ensures that the catalyst cannot dissociate from the polymer chain (and start new chains). This means that the number of polymer chains at the end of the polymerization should be equal to the number of catalysts in solution and that the average degree of polymerization in the sample at the end of polymerization should be equal to the ratio of monomers to catalysts in solution (e.g. if there are 50 equivalents of monomer to one equivalent of catalyst, most of the polymers at the end of polymerization will have 50 repeat units).[7]
Termination
A characteristic of a true CTP is living growth character, meaning that once all monomer has been converted, addition of more monomer to the polymerization should result in monomers being added to the end of polymer chains (rather than starting new chains). This should be true of CTP, but the organometallic nature of the polymer chain end (being capped with the nickel catalyst after C-X oxidative addition), means that if the polymerization is allowed to go to high conversion, disproportionation occurs between two chain ends forming polymers with an integer multiple of the expected molecular weight. For this reason, when CTP is used to make polymers of a specific molecular weight, the polymerization is terminated early by addition of strong acid, iodine, or bromine.

Additionally, if the π-complex is too weakly bound, termination of polymer chains can occur if a chain transfer agent (i.e. solvent, monomer, polymer, any species that can bind to the catalyst) displaces the catalyst from its polymer chain, poisoning the catalyst or starting new chains. This gives polymers with lower than expected average molecular weight.
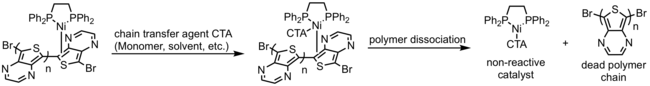
Current research into CTP focuses on finding catalysts that form strong catalyst-polymer π-complexes such that the polymerization remains living.
Analysis
Success of CTP is often evaluated using gel permeation chromatography, matrix-assisted laser desorption/ionization, nuclear magnetic resonance spectroscopy, and gas chromatography. GPC characterization allows for determination of average molecular weight and whether disproportionation has occurred (evidenced by multiple peaks in the GPC trace at different, integer multiple molecular weights of the lowest molecular weight peak). MALDI and NMR allow for identification of end groups of the polymer chain, which can help to elucidate if the polymerization is living. And GC allows for determination of conversion, and can be used in conjunction with other methods to create a molecular weight vs. monomer conversion plot.
Polymer Reactivity versus π-complex
In theory, the chain growth nature of CTP can be described without invoking a catalyst-polymer π-complex. If we assume that no π-complex forms and instead every time a monomer was added to a polymer, the polymer becomes more reactive, we would also see chain growth since the largest polymers in the reaction would be the most reactive and would react with monomers preferentially[8]. The difference between these two explanations of chain-growth can be elucidated by studying the presence of end groups of the polymers using mass spectrometry[9].
Polymers That Can Be Synthesized via CTP
The monomer scope of CTP is limited, but a non-exhaustive list of the polymers that can be synthesized using CTP are below[10]:
- Polythiophene
- Polyphenylene
- Polyselenophene
- Polytellurophene
- Polythiazole
- Polybenzothiadiazole
- Poly(1-hexene)-block-poly(thiophene) (with the 1-hexene block polymerizeed via chain growth, and thiophene polymerized via CTP)
- Polypyrrole
- Polyfluorene
References
- ↑ Tsutomu Yokozawa; Yoshihiro Ohta (2016). "Transformation of Step-Growth Polymerization into Living Chain-Growth Polymerization". Chemical Reviews. 116: 1950–1968. doi:10.1021/acs.chemrev.5b00393.
- ↑ Zachary J. Bryan; Anne J. McNeil (2013). "Conjugated Polymer Synthesis via Catalyst-Transfer Polycondensation (CTP): Mechanism, Scope, and Applications". Macromolecules. 46: 8395–8405. doi:10.1021/ma401314x.
- ↑ Yokoyama, A.; Miyakoshi, R; Yokozawa, T (2004). "Chain-Growth Polymerization for Poly(3-hexylthiophene) with a Defined Molecular Weight and a Low Polydispersity". Macromolecules. 37: 1169–1171. doi:10.1021/ma035396o.
- ↑ Elena E. Sheina; Jinsong Liu; Mihaela Corina Iovu; Darin W. Laird; Richard D. McCullough (2004). "Chain Growth Mechanism for Regioregular Nickel-Initiated Cross-Coupling Polymerizations". Macromolecules. 37: 3526–3528. doi:10.1021/ma0357063.
- ↑ Amanda K. Leone; Anne J. McNeil (2016). "Matchmaking in Catalyst-Transfer Polycondensation: Optimizing Catalysts based on Mechanistic Insight". Accounts of Chemical Research. 49: 2822–2831. doi:10.1021/acs.accounts.6b00488.
- ↑ Zachary J. Bryan; Anne J. McNeil (2013). "Conjugated Polymer Synthesis via Catalyst-Transfer Polycondensation (CTP): Mechanism, Scope, and Applications". Macromolecules. 46: 8395–8405. doi:10.1021/ma401314x.
- ↑ Zachary J. Bryan; Anne J. McNeil (2013). "Conjugated Polymer Synthesis via Catalyst-Transfer Polycondensation (CTP): Mechanism, Scope, and Applications". Macromolecules. 46: 8395–8405. doi:10.1021/ma401314x.
- ↑ Zachary J. Bryan; Ariana O. Hall; Carolyn T. Zhao; Jing Chen; Anne J. McNeil (2016). "Limitations of Using Small Molecules to Identify Catalyst-Transfer Polycondensation Reactions". ACS Macro Letters. 5: 74–77. doi:10.1021/acsmacrolett.5b00746.
- ↑ Kendra D. Souther; Amanda K. Leone; Andrew K. Vitek; Edmund F. Palermo; Anne M. LaPointe; Geoffrey W. Coates; Paul M. Zimmerman; Anne J. McNeil (2017). "Trials and Tribulations of Designing Multitasking Catalysts for Olefin/Thiophene Block Copolymerizations". JOURNAL OF POLYMER SCIENCE, PART A: POLYMER CHEMISTRY. 56: 132–137. doi:10.1002/pola.28885.
- ↑ Amanda K. Leone; Anne J. McNeil (2016). "Matchmaking in Catalyst-Transfer Polycondensation: Optimizing Catalysts based on Mechanistic Insight". Accounts of Chemical Research. 49: 2822–2831. doi:10.1021/acs.accounts.6b00488.