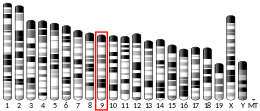
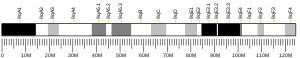
ANLN
Anillin is a conserved protein implicated in cytoskeletal dynamics during cellularization and cytokinesis. The ANLN gene in humans and the scraps gene in Drosophila encode Anillin.[5] In 1989, anillin was first isolated in embryos of Drosophila melanogaster. It was identified as an F-actin binding protein.[6] Six years later, the anillin gene was cloned from cDNA originating from a Drosophila ovary. Staining with anti-anillin (Antigen 8) antibody showed the anillin localizes to the nucleus during interphase and to the contractile ring during cytokinesis.[7] These observations agree with further research that found anillin in high concentrations near the cleavage furrow coinciding with RhoA, a key regulator of contractile ring formation.[8]
The name of the protein anillin originates from a Spanish word, anillo. Anillo means ring and shows that the name anillin references the observed enrichment of anillins at the contractile ring during cytokinesis. Anillins are also enriched at other actomyosin rings, most significantly, those at the leading edge of the Drosophila embryo during cellularization. These actomyosin rings invaginate to separate all nuclei for one another in the syncytial blastoderm.[9]
Structure
Anillin has a unique multi-domain structure. At the N-terminus, there is an actin- and myosin-binding domain. At the C-terminus, there is a PH domain. The PH domain is conserved and essential for anillin functionality.[10] The human anillin cDNA, located on Chr7, encodes a 1,125–amino acid protein with a predicted molecular mass of 124 kD and a pI of 8.1. The mouse anillin gene is located on Chr9.[11]
There are also numerous anillin-like protein homologues found outside of metazoans. In Schizosaccharomyces pombe (fission yeast), there are Mid1p and Mid2p. These two anillin-like proteins do not have any overlap in their functions. Mid1p has been characterized as a key regulator in cytokinesis, responsible for arranging contractile ring assembly and positioning.[12] Mid2p acts later in cytokinesis to organize septins during septation, or the invagination of inner membranes, outer membranes, and the cell wall that occurs in order to separate daughter cells completely.[13] Saccharomyces cerevisiae (budding yeast) also have two anillin-like proteins, Boi1p and Boi2p. Boi1p and Boi2p localize to the nucleus and contractile ring at the bud neck, respectively. They are essential for cell growth and bud formation.[14]

Function
Anillins are required for the faithfulness of cytokinesis and its F-actin-, myosin-, and septin-binding domains implicate anillin in actomyosin cytoskeletal organization. In agreement with this belief, anillin-mutant cells have disrupted contractile rings. Additionally, it is hypothesized that anillin couples the actomyosin cytoskeleton to microtubules by binding MgcRacGAP/CYK-4/RacGAP50C.[15]
Anillins have also been shown to organize the actomyosin cytoskeleton into syncytial structures observed in Drosophila embryos or C. elegans gonads. ANI-1 and ANI-2 (proteins homologous to anillin) are essential for embryonic viability in both organisms. ANI-1 is required for cortical ruffling, pseudocleavage, and all contractile events that occur in embryos prior to mitosis. ANI-1 is also crucial for segregation of polar bodies during meiosis. ANI-2 functions in the maintenance of the structure of the central core of the cytoplasm, the rachis, during oogenesis. ANI-2 ensures oocytes do not disconnect prematurely from the rachis, thereby leading to the generation of embryos of varying sizes.[16]
Binding Partners
One of the best ways to uncover the many functions of anillin is to study the interactions of the protein with its binding partners.
Actin
Anillin specifically binds F-actin, rather than G-actin. Binding of F-actin by anillin only occurs during cell division. Anillin is also bundles actin filaments together. Amino acids 258-340 are sufficient and necessary for F-actin binding in Drosophila, but amino acids 246-371 are necessary to bundle actin filaments.[17] The ability of anillin to bind to and bundle actin together is conversed through many species. It is hypothesized that by regulating actin bundling, anillin increases the efficiency of actomyosin contractility during cell division. Both anillin and F-actin are found in contractile structures. They are recruited independently to the contractile ring, but F-actin increases the efficiency of anillin targeting.[18] Anillin may also be involved in promoting the polymerization of F-actin by stabilizing formin mDia2 in an active form.[19]
Myosin
Anillin interacts directly with non-muscle myosin II and interacts indirectly with myosin via F-actin. Residues 142-254 (near the N-terminus) are essential for anillin binding myosin in Xenopus. The interaction of anillin and myosin is also dependent on phosphorylation of the myosin light chain.[20] The interaction of myosin and anillin does not seem to serve in recruitment, but rather organization of myosin. In Drosophila, anillin is necessary to organize myosin into rings in the cellularization front.[21] Depletion of anillin in Drosophila and humans leads to changes in the spatial and temporal stability of myosin during cytokinesis.[22] In C. elegans, ANI-1 organizes myosin into foci during cytokinesis and establishment of polarity, whereas, ANI-2 is a requirement for the maintenance of myosin-rich contractile lining of oogenic gonads.[23]
Septins
Septin localization during cytokinesis and cellularization is dependent on its association with anillin.[24] The direct interaction between anillin and septins was first shown by the interaction seen between Xenopus anillin and a minimal reconstituted heterooligomer of human septins 2, 6, and 7.[25] The ability of anillin to bind to septins is dependent on the C-terminal domain, which contains a terminal PH domain and an upstream sequence known as the “Anillin Homology” (AH) domain.[26]
Rho
The AH domain of human anillin is essential for its interaction with RhoA. Depletion of RhoA halts contractile ring assembly and ingression, whereas, anillin depletion leads to a less severe phenotype when the contractile ring forms and ingresses partially. Depletion of anillin in Drosophila spermatocytes greatly reduces the localization of Rho and F-actin to equatorial regions.[27]
Ect2
Anillin interacts with Ect2, further supporting the idea that anillin stabilizes RhoA localization since Ect2 is an activator of RhoA. Independent of RhoA, the interaction between anillin and Ect2 occurs. This interaction is essential of the GEF activity of Ect2 and requires the AH domain of anillin and the PH domain of Ect2.[28]
Cyk-4
Drosophila anillin interacts with Cyk-4, a central spindle protein, indicating that anillin may have a role in determining the division plane during cytokinesis.[29] In anillin-depleted larval cells, the central spindle does not extend to the cortex.[30] Human anillin-depleted cells show improperly positioned and distorted central spindles.[31]
Microtubules
Anillin was first isolated from Drosophila by harnessing its interactions with both F-actin and microtubules.[32] Furthermore, anillin-rich structures that form after Latrunculin A treatment of Drosophila cells localize to the plus-ends of microtubules.[33] The interaction between anillin and microtubules suggest that anillin may serve as a signaling factor to relay the position of the mitotic spindle to the cortex to ensure appropriate contractile ring formation during cytokinesis.[34]
Regulation
Anillins in metazoans are heavily phosphorylated; however, the kinases responsible for the phosphorylation are unknown at the present time. In humans and Drosophila, anillins are recruited to the equatorial cortex in a RhoA-dependent manner. This recruitment is independent of other cytoskeletal Rho targets such as myosin, F-actin, and Rho-kinase. It has been observed that anillin proteolysis is triggered after mitotic exit by the Anaphase Promoting Complex (APC).
Most anillins can be sequestered to the nucleus during interphase, but there are exceptions – Drosophila anilins in the early embryo, C. elegans ANI-1 in early embryos, C. elegans ANI-2 in oogenic gonads, and Mid2p in fission yeast. These anillins that are not sequestered during interphase suggest that anillins may also regulate cytoskeletal dynamics outside the contractile ring during cytokinesis.[35]
Role in Diseases
Anillin is critical for cell division and therefore development and homeostasis in metazoans. In recent years, the expression levels of anillin have been shown to correlate to the metastatic potential of human tumours. In colorectal cancer, expression levels of anillin are higher in tumours and when anillin was over-expressed in HT29 cells, a classical colorectal cancer cell line, the cells showed faster replication kinetics due to the lengthening of G2/M phase. Increasing the expression of anillin also led to further invasiveness and migration of numerous colorectal cancer cell lines. The hypothesis from such observations is that anillin promotes EMT and cell migration through cytoskeletal remodeling, leading to enhanced proliferation, invasion, and mobility of tumour cells.[36]
References
- 1 2 3 GRCh38: Ensembl release 89: ENSG00000011426 - Ensembl, May 2017
- 1 2 3 GRCm38: Ensembl release 89: ENSMUSG00000036777 - Ensembl, May 2017
- ↑ "Human PubMed Reference:".
- ↑ "Mouse PubMed Reference:".
- ↑ Piekny, A. J., & Maddox, A. S. (2010). The myriad roles of Anillin during cytokinesis. Semin Cell Dev Biol, 21(9), 881-891. doi: 10.1016/j.semcdb.2010.08.002
- ↑ Zhang, L., & Maddox, A. S. (2010). Anillin. Curr Biol, 20(4), 135-136. doi: 10.1016/j.cub.2009.12.017
- ↑ Field, C. M., & Alberts, B. M. (1995). Anillin, a Contractile Ring Protein That Cycles from the Nucleus to the Cell Cortex. The Journal of Cell Biology, 131(1), 165-178.
- ↑ Piekny, A. J., & Glotzer, M. (2008). Anillin is a scaffold protein that links RhoA, actin, and myosin during cytokinesis. Curr Biol, 18(1), 30-36. doi: 10.1016/j.cub.2007.11.068
- ↑ Piekny, A. J., & Maddox, A. S. (2010). The myriad roles of Anillin during cytokinesis. Semin Cell Dev Biol, 21(9), 881-891. doi: 10.1016/j.semcdb.2010.08.002
- ↑ Piekny, A. J., & Glotzer, M. (2008). Anillin is a scaffold protein that links RhoA, actin, and myosin during cytokinesis. Curr Biol, 18(1), 30-36. doi: 10.1016/j.cub.2007.11.068
- ↑ Oegema, K., Savovian, M. S., Mitchison, T. J., & Field, C. M. (2000). Functional Analysis of a Human Homologue of the Drosophila Actin Binding Protein Anillin Suggest a Role in Cytokinesis. The Journal of Cell Biology, 150(3), 539-551.
- ↑ Saha, S., & Pollard, T. D. (2012). Characterization of structural and functional domains of the anillin-related protein Mid1p that contribute to cytokinesis in fission yeast. Mol Biol Cell, 23(20), 3993-4007. doi: 10.1091/mbc.E12-07-0536
- ↑ Tasto, J. J., Morrell, J. L., & Gould, K. L. (2003). An anillin homologue, Mid2p, acts during fission yeast cytokinesis to organize the septin ring and promote cell separation. J Cell Biol, 160(7), 1093-1103. doi: 10.1083/jcb.200211126
- ↑ Toya, M., Iino, Y., & Yamamoto, M. (1999). Fission Yeast Pob1p, Which Is Homologous to Budding Yeast Boi Proteins and Exhibits Subcellular Localization Close to Actin Patches, Is Essential for Cell Elongation and Separation. Mol Biol Cell, 10(8), 2745-2757.
- ↑ D'Avino, P. P., Takeda, T., Capalbo, L., Zhang, W., Lilley, K. S., Laue, E. D., & Glover, D. M. (2008). Interaction between Anillin and RacGAP50C connects the actomyosin contractile ring with spindle microtubules at the cell division site. J Cell Sci, 121(Pt 8), 1151-1158. doi: 10.1242/jcs.026716
- ↑ Maddox, A. S., Habermann, B., Desai, A., & Oegema, K. (2005). Distinct roles for two C. elegans anillins in the gonad and early embryo. Development, 132(12), 2837-2848. doi: 10.1242/dev.01828
- ↑ Field, C. M., & Alberts, B. M. (1995). Anillin, a Contractile Ring Protein That Cycles from the Nucleus to the Cell Cortex. The Journal of Cell Biology, 131(1), 165-178.
- ↑ Piekny, A. J., & Maddox, A. S. (2010). The myriad roles of Anillin during cytokinesis. Semin Cell Dev Biol, 21(9), 881-891. doi: 10.1016/j.semcdb.2010.08.002
- ↑ Watanabe, S., Okawa, K., Miki, T., Sakamoto, S., Morinaga, T., Segawa, K., et al. (2010). Rho and Anillin-dependent control of mDia2 localization and function in cytokinesis. Mol Biol Cell, 21(18), 3193-3204. doi: 10.1091/mbc.E10-04-0324
- ↑ Straight A. F., F., C. M., Mitchison, T. J. (2005). Anillin binds nonmuscle myosin II and regulates the contractile ring. Mol Biol Cell, 16(1), 193–201. doi: 10.1091/mbc.E04-08-0758
- ↑ Field, C. M., Coughlin, M., Doberstein, S., Marty, T., & Sullivan, W. (2005). Characterization of anillin mutants reveals essential roles in septin localization and plasma membrane integrity. Development, 132(12), 2849-2860. doi: 10.1242/dev.01843
- ↑ Goldbach, P., Wong, R., Beise, N., Sarpal, R., Trimble, W. S., & Brill, J. A. (2010). Stabilization of the Actomyosin Ring Enables Spermatocyte Cytokinesis in Drosophila. Mol Biol Cell, 21(9). doi: 10.1091/mbc.E09-08-0714
- ↑ Maddox, A. S., Habermann, B., Desai, A., & Oegema, K. (2005). Distinct roles for two C. elegans anillins in the gonad and early embryo. Development, 132(12), 2837-2848. doi: 10.1242/dev.01828
- ↑ Versele, M., & Thorner, J. (2005). Some assembly required: yeast septins provide the instruction manual. Trends Cell Biol, 15(8), 414-424. doi: 10.1016/j.tcb.2005.06.007
- ↑ Kinoshita, M., Field, C. M., Coughlin, M. L., Straight, A. F., & Mitchison, T. J. (2002). Self- and Actin-Templated Assembly of Mammalian Septins. Developmental Cell, 3(6), 791-802. doi: 10.1016/S1534-5807(02)00366-0
- ↑ Oegema, K., Savovian, M. S., Mitchison, T. J., & Field, C. M. (2000). Functional Analysis of a Human Homologue of the Drosophila Actin Binding Protein Anillin Suggest a Role in Cytokinesis. The Journal of Cell Biology, 150(3), 539-551.
- ↑ Goldbach, P., Wong, R., Beise, N., Sarpal, R., Trimble, W. S., & Brill, J. A. (2010). Stabilization of the Actomyosin Ring Enables Spermatocyte Cytokinesis in Drosophila. Mol Biol Cell, 21(9). doi: 10.1091/mbc.E09-08-0714
- ↑ Solski, P. A., Wilder, R. S., Rossman, K. L., Sondek, J., Cox, A. D., Campbell, S. L., & Der, C. J. (2004). Requirement for C-terminal sequences in regulation of Ect2 guanine nucleotide exchange specificity and transformation. J Biol Chem, 279(24), 25226-25233. doi: 10.1074/jbc.M313792200
- ↑ Glotzer, M. (2009). The 3Ms of central spindle assembly: microtubules, motors and MAPs. Nat Rev Mol Cell Biol, 10(1), 9-20. doi: 10.1038/nrm2609
- ↑ Gregory, S. L., Ebrahimi, S., Milverton, J., Jones, W. M., Bejsovec, A., & Saint, R. (2008). Cell division requires a direct link between microtubule-bound RacGAP and Anillin in the contractile ring. Curr Biol, 18(1), 25-29. doi: 10.1016/j.cub.2007.11.050
- ↑ Zhao, W. M., & Fang, G. (2005). Anillin is a substrate of anaphase-promoting complex/cyclosome (APC/C) that controls spatial contractility of myosin during late cytokinesis. J Biol Chem, 280(39), 33516-33524. doi: 10.1074/jbc.M504657200
- ↑ Sisson, J. C., Field, C., Ventura, R., Royou, A., & Sullivan, W. (2000). Lava Lamp, a Novel Peripheral Golgi Protein, Is Required for Drosophila melanogaster Cellularization. The Journal of Cell Biology, 151(4), 905-917. doi: 10.1083/jcb.151.4.905
- ↑ Hickson, G. R. X., & O'Farrell, P. H. (2008). Rho-dependent control of anillin behavior during cytokinesis. JCB, 180(2), 285-294. doi: 10.1083/jcb.200709005
- ↑ Piekny, A. J., & Maddox, A. S. (2010). The myriad roles of Anillin during cytokinesis. Semin Cell Dev Biol, 21(9), 881-891. doi: 10.1016/j.semcdb.2010.08.002
- ↑ Zhang, L., & Maddox, A. S. (2010). Anillin. Curr Biol, 20(4), 135-136. doi: 10.1016/j.cub.2009.12.017
- ↑ Chuang, H. Y., & Ou, Y. H. (2014). Overexpression of anillin in colorectal cancer promoter the cell proliferation, cell mobility and cell invasion. Paper presented at the Proceedings of the 105th Annual Meeting of the American Association for Cancer Research, San Diego, CA.
External links
- Human ANLN genome location and ANLN gene details page in the UCSC Genome Browser.
Further reading
- Strausberg RL, Feingold EA, Grouse LH, et al. (2003). "Generation and initial analysis of more than 15,000 full-length human and mouse cDNA sequences". Proc. Natl. Acad. Sci. U.S.A. 99 (26): 16899–903. doi:10.1073/pnas.242603899. PMC 139241. PMID 12477932.
- Kinoshita M, Field CM, Coughlin ML, et al. (2003). "Self- and actin-templated assembly of Mammalian septins". Dev. Cell. 3 (6): 791–802. doi:10.1016/S1534-5807(02)00366-0. PMID 12479805.
- Straight AF, Cheung A, Limouze J, et al. (2003). "Dissecting temporal and spatial control of cytokinesis with a myosin II Inhibitor". Science. 299 (5613): 1743–7. doi:10.1126/science.1081412. PMID 12637748.
- Ota T, Suzuki Y, Nishikawa T, et al. (2004). "Complete sequencing and characterization of 21,243 full-length human cDNAs". Nat. Genet. 36 (1): 40–5. doi:10.1038/ng1285. PMID 14702039.
- Beausoleil SA, Jedrychowski M, Schwartz D, et al. (2004). "Large-scale characterization of HeLa cell nuclear phosphoproteins". Proc. Natl. Acad. Sci. U.S.A. 101 (33): 12130–5. doi:10.1073/pnas.0404720101. PMC 514446. PMID 15302935.
- Gerhard DS, Wagner L, Feingold EA, et al. (2004). "The status, quality, and expansion of the NIH full-length cDNA project: the Mammalian Gene Collection (MGC)". Genome Res. 14 (10B): 2121–7. doi:10.1101/gr.2596504. PMC 528928. PMID 15489334.
- Straight AF, Field CM, Mitchison TJ (2005). "Anillin binds nonmuscle myosin II and regulates the contractile ring". Mol. Biol. Cell. 16 (1): 193–201. doi:10.1091/mbc.E04-08-0758. PMC 539163. PMID 15496454.
- Mollinari C, Kleman JP, Saoudi Y, et al. (2005). "Ablation of PRC1 by small interfering RNA demonstrates that cytokinetic abscission requires a central spindle bundle in mammalian cells, whereas completion of furrowing does not". Mol. Biol. Cell. 16 (3): 1043–55. doi:10.1091/mbc.E04-04-0346. PMC 551472. PMID 15616196.
- Andersen JS, Lam YW, Leung AK, et al. (2005). "Nucleolar proteome dynamics". Nature. 433 (7021): 77–83. doi:10.1038/nature03207. PMID 15635413.
- Monzo P, Gauthier NC, Keslair F, et al. (2005). "Clues to CD2-associated protein involvement in cytokinesis". Mol. Biol. Cell. 16 (6): 2891–902. doi:10.1091/mbc.E04-09-0773. PMC 1142433. PMID 15800069.
- Zhao WM, Fang G (2005). "Anillin is a substrate of anaphase-promoting complex/cyclosome (APC/C) that controls spatial contractility of myosin during late cytokinesis". J. Biol. Chem. 280 (39): 33516–24. doi:10.1074/jbc.M504657200. PMID 16040610.
- Zhao WM, Fang G (2005). "MgcRacGAP controls the assembly of the contractile ring and the initiation of cytokinesis". Proc. Natl. Acad. Sci. U.S.A. 102 (37): 13158–63. doi:10.1073/pnas.0504145102. PMC 1201590. PMID 16129829.
- Hall PA, Todd CB, Hyland PL, et al. (2006). "The septin-binding protein anillin is overexpressed in diverse human tumors". Clin. Cancer Res. 11 (19 Pt 1): 6780–6. doi:10.1158/1078-0432.CCR-05-0997. PMID 16203764.
- Suzuki C, Daigo Y, Ishikawa N, et al. (2006). "ANLN plays a critical role in human lung carcinogenesis through the activation of RHOA and by involvement in the phosphoinositide 3-kinase/AKT pathway". Cancer Res. 65 (24): 11314–25. doi:10.1158/0008-5472.CAN-05-1507. PMID 16357138.
- Beausoleil SA, Villén J, Gerber SA, et al. (2006). "A probability-based approach for high-throughput protein phosphorylation analysis and site localization". Nat. Biotechnol. 24 (10): 1285–92. doi:10.1038/nbt1240. PMID 16964243.
- Olsen JV, Blagoev B, Gnad F, et al. (2006). "Global, in vivo, and site-specific phosphorylation dynamics in signaling networks". Cell. 127 (3): 635–48. doi:10.1016/j.cell.2006.09.026. PMID 17081983.