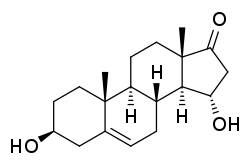
15α-Hydroxy-DHEA
 | |
| Names | |
|---|---|
| IUPAC name
(3S,8R,9S,10R,13S,14S,15S)-3,15-Dihydroxy-10,13-dimethyl-1,2,3,4,7,8,9,11,12,14,15,16-dodecahydrocyclopenta[a]phenanthren-17-one | |
| Other names
3β,15α-Dihydroxyandrost-5-en-17-one; 15α-Hydroxydehydroepiandrosterone; 15α-Hydroxy-DHEA; 15α-OH-DHEA | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
PubChem CID |
|
| |
| |
| Properties | |
| C19H28O3 | |
| Molar mass | 304.43 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
15α-Hydroxydehydroepiandrosterone, abbreviated as 15α-hydroxy-DHEA or 15α-OH-DHEA, is an endogenous metabolite of dehydroepiandrosterone (DHEA).[1][2][3] Both 15α-OH-DHEA and its 3β-sulfate ester, 15α-OH-DHEA-S, are intermediates in the biosynthesis of estetrol from dehydroepiandrosterone (DHEA).[1][2][3]
See also
References
- 1 2 Roger Smith (Prof.) (1 January 2001). The Endocrinology of Parturition: Basic Science and Clinical Application. Karger Medical and Scientific Publishers. pp. 91–. ISBN 978-3-8055-7195-1.
- 1 2 J.B. Josimovich (11 November 2013). Gynecologic Endocrinology. Springer Science & Business Media. pp. 32–. ISBN 978-1-4613-2157-6.
- 1 2 Jerome F. Strauss, III; Robert L. Barbieri (13 September 2013). Yen and Jaffe's Reproductive Endocrinology. Elsevier Health Sciences. pp. 257–. ISBN 978-1-4557-2758-2.
This article is issued from
Wikipedia.
The text is licensed under Creative Commons - Attribution - Sharealike.
Additional terms may apply for the media files.