CNO cycle
The CNO cycle (for carbon–nitrogen–oxygen) is one of the two known sets of fusion reactions by which stars convert hydrogen to helium, the other being the proton–proton chain reaction (pp-chain reaction). Unlike the latter, the CNO cycle is a catalytic cycle. It is dominant in stars that are more than 1.3 times as massive as the Sun.[1]
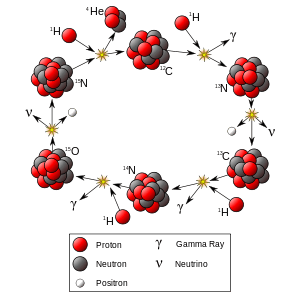
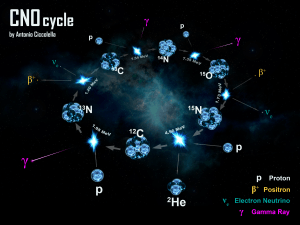
In the CNO cycle, four protons fuse, using carbon, nitrogen, and oxygen isotopes as catalysts, to produce one alpha particle, two positrons and two electron neutrinos. Although there are various paths and catalysts involved in the CNO cycles, all these cycles have the same net result:
- 4 1
1H
+ 2
e−
→ 4
2He
+ 2
e+
+ 2
e−
+ 2
ν
e + 3
γ
+ 24.7 MeV → 4
2He
+ 2
ν
e + 7
γ
+ 26.7 MeV
The positrons will almost instantly annihilate with electrons, releasing energy in the form of gamma rays. The neutrinos escape from the star carrying away some energy. One nucleus goes on to become carbon, nitrogen, and oxygen isotopes through a number of transformations in an endless loop.
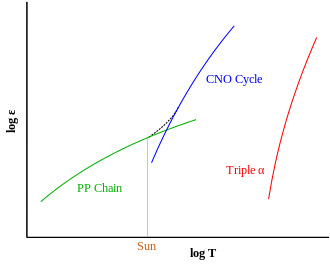
The proton–proton chain is more prominent in stars the mass of the Sun or less. This difference stems from temperature dependency differences between the two reactions; pp-chain reaction starts at temperatures around 4×106 K[2] (4 megakelvin), making it the dominant energy source in smaller stars. A self-maintaining CNO chain starts at approximately 15×106 K, but its energy output rises much more rapidly with increasing temperatures[1] so that it becomes the dominant source of energy at approximately 17×106 K.[3]
The Sun has a core temperature of around 15.7×106 K, and only 1.7% of 4
He
nuclei produced in the Sun are
born in the CNO cycle. The CNO-I process was independently proposed by Carl von Weizsäcker[4][5] and Hans Bethe[6][7] in the late 1930s.
Cold CNO cycles
Under typical conditions found in stars, catalytic hydrogen burning by the CNO cycles is limited by proton captures. Specifically, the timescale for beta decay of the radioactive nuclei produced is faster than the timescale for fusion. Because of the long timescales involved, the cold CNO cycles convert hydrogen to helium slowly, allowing them to power stars in quiescent equilibrium for many years.
CNO-I
The first proposed catalytic cycle for the conversion of hydrogen into helium was initially called the carbon–nitrogen cycle (CN-cycle), also referred to as the Bethe–Weizsäcker cycle in honor of the independent work of Carl von Weizsäcker in 1937-38[4][5] and Hans Bethe. Bethe's 1939 papers on the CN-cycle[6][7] drew on three earlier papers written in collaboration with Robert Bacher and Milton Stanley Livingston[8][9][10] and which came to be known informally as "Bethe's Bible." It was considered the standard work on nuclear physics for many years and was a significant factor in his being awarded the 1967 Nobel Prize in Physics.[11] Bethe's original calculations suggested the CN-cycle was the Sun's primary source of energy.[6][7] This conclusion arose from what is now-known as a mistaken belief: that the abundance of nitrogen in the sun is approximately 10%, when it is actually less than half a percent.[12] The CN-cycle, named as it contains no stable isotope of oxygen involves the following cycle of transformations: 12
6C
→ 13
7N
→ 13
6C
→ 14
7N
→ 15
8O
→ 15
7N
→ 12
6C
.[12] This cycle is now understood as being the first part of a larger process, the CNO-cycle, and the main reactions in this part of the cycle (CNO-I) are:[12]
12
6C
+ 1
1H
→ 13
7N
+
γ
+ 1.95 MeV 13
7N
→ 13
6C
+
e+
+
ν
e+ 1.20 MeV (half-life of 9.965 minutes[13]) 13
6C
+ 1
1H
→ 14
7N
+
γ
+ 7.54 MeV 14
7N
+ 1
1H
→ 15
8O
+
γ
+ 7.35 MeV 15
8O
→ 15
7N
+
e+
+
ν
e+ 1.73 MeV (half-life of 2.034 minutes[13]) 15
7N
+ 1
1H
→ 12
6C
+ 4
2He
+ 4.96 MeV
where the carbon-12 nucleus used in the first reaction is regenerated in the last reaction. After the two positrons emitted annihilate with two ambient electrons producing an additional 2.04 MeV, the total energy released in one cycle is 26.73 MeV; in some texts, authors are erroneously including the positron annihilation energy in with the beta-decay Q-value and then neglecting the equal amount of energy released by annihilation, leading to possible confusion. All values are calculated with reference to the Atomic Mass Evaluation 2003.[14]
The limiting (slowest) reaction in the CNO-I cycle is the proton capture on 14
7N
. In 2006 it was experimentally measured down to stellar energies, revising the calculated age of globular clusters by around 1 billion years.[15]
The neutrinos emitted in beta decay will have a spectrum of energy ranges, because although momentum is conserved, the momentum can be shared in any way between the positron and neutrino, with either emitted at rest and the other taking away the full energy, or anything in between, so long as all the energy from the Q-value is used. The total momentum received by the electron and the neutrino is not great enough to cause a significant recoil of the much heavier daughter nucleus and hence, its contribution to kinetic energy of the products, for the precision of values given here, can be neglected. Thus the neutrino emitted during the decay of nitrogen-13 can have an energy from zero up to 1.20 MeV, and the neutrino emitted during the decay of oxygen-15 can have an energy from zero up to 1.73 MeV. On average, about 1.7 MeV of the total energy output is taken away by neutrinos for each loop of the cycle, leaving about 25 MeV available for producing luminosity.[16]
CNO-II
In a minor branch of the above reaction, occurring in the Sun's core 0.04% of the time, the final reaction involving 15
7N
shown above does not produce carbon-12 and an alpha particle, but instead produces oxygen-16 and a photon and continues 15
7N
→16
8O
→17
9F
→17
8O
→14
7N
→15
8O
→15
7N
:
15
7N
+ 1
1H
→ 16
8O
+
γ
+ 12.13 MeV 16
8O
+ 1
1H
→ 17
9F
+
γ
+ 0.60 MeV 17
9F
→ 17
8O
+
e+
+
ν
e+ 2.76 MeV (half-life of 64.49 seconds) 17
8O
+ 1
1H
→ 14
7N
+ 4
2He
+ 1.19 MeV 14
7N
+ 1
1H
→ 15
8O
+
γ
+ 7.35 MeV 15
8O
→ 15
7N
+
e+
+
ν
e+ 2.75 MeV (half-life of 122.24 seconds)
Like the carbon, nitrogen, and oxygen involved in the main branch, the fluorine produced in the minor branch is merely an intermediate product and at steady state, does not accumulate in the star.
CNO-III
This subdominant branch is significant only for massive stars. The reactions are started when one of the reactions in CNO-II results in fluorine-18 and gamma instead of nitrogen-14 and alpha, and continues 17
8O
→18
9F
→18
8O
→15
7N
→16
8O
→17
9F
→17
8O
:
17
8O
+ 1
1H
→ 18
9F
+
γ
+ 5.61 MeV 18
9F
→ 18
8O
+
e+
+
ν
e+ 1.656 MeV (half-life of 109.771 minutes) 18
8O
+ 1
1H
→ 15
7N
+ 4
2He
+ 3.98 MeV 15
7N
+ 1
1H
→ 16
8O
+
γ
+ 12.13 MeV 16
8O
+ 1
1H
→ 17
9F
+
γ
+ 0.60 MeV 17
9F
→ 17
8O
+
e+
+
ν
e+ 2.76 MeV (half-life of 64.49 seconds)
CNO-IV
Like the CNO-III, this branch is also only significant in massive stars. The reactions are started when one of the reactions in CNO-III results in fluorine-19 and gamma instead of nitrogen-15 and alpha, and continues 18
8O
→19
9F
→16
8O
→17
9F
→17
8O
→18
9F
→18
8O
:
18
8O
+ 1
1H
→ 19
9F
+
γ
+ 7.994 MeV 19
9F
+ 1
1H
→ 16
8O
+ 4
2He
+ 8.114 MeV 16
8O
+ 1
1H
→ 17
9F
+
γ
+ 0.60 MeV 17
9F
→ 17
8O
+
e+
+
ν
e+ 2.76 MeV (half-life of 64.49 seconds) 17
8O
+ 1
1H
→ 18
9F
+
γ
+ 5.61 MeV 18
9F
→ 18
8O
+
e+
+
ν
e+ 1.656 MeV (half-life of 109.771 minutes)
In some instances F-18 can combine with a helium nucleus to start a sodium-neon cycle[17]
Hot CNO cycles
Under conditions of higher temperature and pressure, such as those found in novae and x-ray bursts, the rate of proton captures exceeds the rate of beta-decay, pushing the burning to the proton drip line. The essential idea is that a radioactive species will capture a proton before it can beta decay, opening new nuclear burning pathways that are otherwise inaccessible. Because of the higher temperatures involved, these catalytic cycles are typically referred to as the hot CNO cycles; because the timescales are limited by beta decays instead of proton captures, they are also called the beta-limited CNO cycles.
HCNO-I
The difference between the CNO-I cycle and the HCNO-I cycle is that 13
7N
captures a proton instead of decaying, leading to the total sequence 12
6C
→13
7N
→14
8O
→14
7N
→15
8O
→15
7N
→12
6C
:
HCNO-II
The notable difference between the CNO-II cycle and the HCNO-II cycle is that 17
9F
captures a proton instead of decaying, and neon is produced in a subsequent reaction on 18
9F
, leading to the total sequence 15
7N
→16
8O
→17
9F
→18
10Ne
→18
9F
→15
8O
→15
7N
:
15
7N
+ 1
1H
→ 16
8O
+
γ
+ 12.13 MeV 16
8O
+ 1
1H
→ 17
9F
+
γ
+ 0.60 MeV 17
9F
+ 1
1H
→ 18
10Ne
+
γ
+ 3.92 MeV 18
10Ne
→ 18
9F
+
e+
+
ν
e+ 4.44 MeV (half-life of 1.672 seconds) 18
9F
+ 1
1H
→ 15
8O
+ 4
2He
+ 2.88 MeV 15
8O
→ 15
7N
+
e+
+
ν
e+ 2.75 MeV (half-life of 122.24 seconds)
HCNO-III
An alternative to the HCNO-II cycle is that 18
9F
captures a proton moving towards higher mass and using the same helium production mechanism as the CNO-IV cycle as 18
9F
→19
10Ne
→19
9F
→16
8O
→17
9F
→18
10Ne
→18
9F
:
18
9F
+ 1
1H
→ 19
10Ne
+
γ
+ 6.41 MeV 19
10Ne
→ 19
9F
+
e+
+
ν
e+ 3.32 MeV (half-life of 17.22 seconds) 19
9F
+ 1
1H
→ 16
8O
+ 4
2He
+ 8.11 MeV 16
8O
+ 1
1H
→ 17
9F
+
γ
+ 0.60 MeV 17
9F
+ 1
1H
→ 18
10Ne
+
γ
+ 3.92 MeV 18
10Ne
→ 18
9F
+
e+
+
ν
e+ 4.44 MeV (half-life of 1.672 seconds)
Use in astronomy
While the total number of "catalytic" nuclei are conserved in the cycle, in stellar evolution the relative proportions of the nuclei are altered. When the cycle is run to equilibrium, the ratio of the carbon-12/carbon-13 nuclei is driven to 3.5, and nitrogen-14 becomes the most numerous nucleus, regardless of initial composition. During a star's evolution, convective mixing episodes moves material, within which the CNO cycle has operated, from the star's interior to the surface, altering the observed composition of the star. Red giant stars are observed to have lower carbon-12/carbon-13 and carbon-12/nitrogen-14 ratios than do main sequence stars, which is considered to be convincing evidence for the operation of the CNO cycle.
See also
- Stellar nucleosynthesis, the whole topic
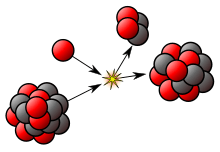
- Triple-alpha process, how 12
C
is produced from lighter nuclei
References
- Salaris, Maurizio; Cassisi, Santi (2005). Evolution of Stars and Stellar Populations. John Wiley and Sons. pp. 119–121. ISBN 0-470-09220-3.
- Reid, I. Neill; Hawley, Suzanne L. (2005). "The Structure, Formation and Evolution of Low-Mass Stars and Brown Dwarfs – Energy Generation". New Light on Dark Stars: Red Dwarfs, Low-Mass Stars, Brown Dwarfs. Springer-Praxis Books in Astrophysics and Astronomy (2nd ed.). Springer Science & Business Media. p. 108–111. ISBN 3-540-25124-3.
- Schuler, S. C.; King, J. R.; The, L.-S. (2009). "Stellar Nucleosynthesis in the Hyades Open Cluster". The Astrophysical Journal. 701 (1): 837–849. arXiv:0906.4812. Bibcode:2009ApJ...701..837S. doi:10.1088/0004-637X/701/1/837.
- von Weizsäcker, Carl F. (1937). "Über Elementumwandlungen in Innern der Sterne I" [On Transformations of Elements in the Interiors of Stars I]. Physikalische Zeitschrift. 38: 176–191.
- von Weizsäcker, Carl F. (1938). "Über Elementumwandlungen in Innern der Sterne II" [On Transformations of Elements in the Interiors of Stars II]. Physikalische Zeitschrift. 39: 633–646.
- Bethe, Hans A. (1939). "Energy Production in Stars". Physical Review. 55 (1): 103. doi:10.1103/PhysRev.55.103.
- Bethe, Hans A. (1939). "Energy Production in Stars". Physical Review. 55 (5): 434–456. doi:10.1103/PhysRev.55.434.
- Bethe, Hans A.; Bacher, Robert (1936). "Nuclear Physics, A: Stationary States of Nuclei" (PDF). Reviews of Modern Physics. 8 (2): 82–229. doi:10.1103/RevModPhys.8.82.
- Bethe, Hans A. (1937). "Nuclear Physics, B: Nuclear Dynamics, Theoretical". Reviews of Modern Physics. 9 (2): 69–244. doi:10.1103/RevModPhys.9.69.
- Bethe, Hans A.; Livingston, Milton S. (1937). "Nuclear Physics, C: Nuclear Dynamics, Experimental". Reviews of Modern Physics. 9 (2): 245–390. doi:10.1103/RevModPhys.9.245.
- Bardi, Jason Socrates (23 January 2008). "Landmarks: What Makes the Stars Shine?". Physical Review Focus. 21 (3). Retrieved 26 November 2018.
- Krane, Kenneth S. (1988). Introductory Nuclear Physics. John Wiley & Sons. p. 537. ISBN 0-471-80553-X.
- Ray, Alak (2010). "Massive Stars as Thermonuclear Reactors and their Explosions Following Core Collapse". In Goswami, Aruna; Reddy, B. Eswar (eds.). Principles and Perspectives in Cosmochemistry. Springer Science & Business Media. p. 233. ISBN 9783642103681.
- Wapstra, Aaldert; Audi, Georges (18 November 2003). "The 2003 Atomic Mass Evaluation". Atomic Mass Data Center. Retrieved 25 October 2011.
- LUNA Collaboration; Lemut, A.; Bemmerer, D.; Confortola, F.; Bonetti, R.; Broggini, C.; Corvisiero, P.; Costantini, H.; Cruz, J.; Formicola, A.; Fülöp, Zs.; Gervino, G.; et al. (2006). "First measurement of the 14N(p,γ)15O cross section down to 70 keV". Physics Letters B. 634: 483–487. arXiv:nucl-ex/0602012. Bibcode:2006PhLB..634..483L. doi:10.1016/j.physletb.2006.02.021.
- Scheffler, Helmut; Elsässer, Hans (1990). Die Physik der Sterne und der Sonne [The Physics of the Stars and the Sun]. Bibliographisches Institut (Mannheim, Wien, Zürich). ISBN 3-411-14172-7.
- https://core.ac.uk/download/pdf/31144835.pdf
Further reading
- Bethe, H. A. (1939). "Energy Production in Stars". Physical Review. 55 (5): 434–56. Bibcode:1939PhRv...55..434B. doi:10.1103/PhysRev.55.434.
- Iben, I. (1967). "Stellar Evolution Within and off the Main Sequence". Annual Review of Astronomy and Astrophysics. 5: 571–626. Bibcode:1967ARA&A...5..571I. doi:10.1146/annurev.aa.05.090167.003035.