Aragonite
Aragonite is a carbonate mineral, one of the three most common naturally occurring crystal forms of calcium carbonate, CaCO3 (the other forms being the minerals calcite and vaterite). It is formed by biological and physical processes, including precipitation from marine and freshwater environments.
| Aragonite | |
|---|---|
 Aragonite from Salsigne mine, Salsigne, Aude, France - Size: 30x30x20 cm | |
| General | |
| Category | Carbonate mineral |
| Formula (repeating unit) | CaCO3 |
| Strunz classification | 5.AB.15 |
| Crystal system | Orthorhombic |
| Crystal class | Dipyramidal (mmm) H-M symbol: (2/m 2/m 2/m) |
| Space group | Pmcn |
| Unit cell | a = 4.95, b = 7.96 c = 5.74 [Å]; Z = 4 |
| Identification | |
| Color | White, red, yellow, orange, green, purple, grey, blue and brown |
| Crystal habit | Pseudohexagonal, prismatic crystals, acicular, columnar, globular, reniform, pisolitic, coralloidal, stalactitic, internally banded |
| Twinning | Polysynthetic parallel to {100} cyclically on {110} |
| Cleavage | Distinct on {010}, imperfect {110} and {011} |
| Fracture | Subconchoidal |
| Tenacity | Brittle |
| Mohs scale hardness | 3.5-4 |
| Luster | Vitreous, resinous on fracture surfaces |
| Streak | White |
| Diaphaneity | Translucent to transparent |
| Specific gravity | 2.95 |
| Optical properties | Biaxial (-) |
| Refractive index | nα = 1.529 - 1.530 nβ = 1.680 - 1.682 nγ = 1.685 - 1.686 |
| Birefringence | δ = 0.156 |
| 2V angle | 18° |
| Solubility | Dilute acid |
| Other characteristics | Fluorescence: pale rose, yellow, white or bluish; phosphorescence: greenish or white (LW UV); yellowish (SW UV) |
| References | [1][2][3] |
The crystal lattice of aragonite differs from that of calcite, resulting in a different crystal shape, an orthorhombic crystal system with acicular crystal. Repeated twinning results in pseudo-hexagonal forms. Aragonite may be columnar or fibrous, occasionally in branching stalactitic forms called flos-ferri ("flowers of iron") from their association with the ores at the Carinthian iron mines.
Occurrence
The type location for aragonite is Molina de Aragón in the Province of Guadalajara in Castilla-La Mancha, Spain, for which it was named in 1797. The mineral is not (as often assumed) named for the region of Aragon: Molina de Aragón is located in the historic region of Castile, albeit only 25 kilometers away from the border with Aragon. Aragonite is found in this locality as cyclic twins inside gypsum and marls of the Keuper facies of Triassic.[4] This type of aragonite deposits are very common in Spain, and there are also some in France and Morocco.
An aragonite cave, the Ochtinská Aragonite Cave, is situated in Slovakia. In the US, aragonite in the form of stalactites and "cave flowers" (anthodite) is known from Carlsbad Caverns and other caves. Massive deposits of oolitic aragonite sand are found on the seabed in the Bahamas.
Aragonite is the high pressure polymorph of calcium carbonate. As such, it occurs in high pressure metamorphic rocks such as those formed at subduction zones.
Aragonite forms naturally in almost all mollusk shells, and as the calcareous endoskeleton of warm- and cold-water corals (Scleractinia). Several serpulids have aragonitic tubes. Because the mineral deposition in mollusk shells is strongly biologically controlled, some crystal forms are distinctively different from those of inorganic aragonite. In some mollusks, the entire shell is aragonite; in others, aragonite forms only discrete parts of a bimineralic shell (aragonite plus calcite). The nacreous layer of the aragonite fossil shells of some extinct ammonites forms an iridescent material called ammolite.
Aragonite also forms in the ocean and in caves as inorganic precipitates called marine cements and speleothems, respectively. Aragonite is not uncommon in serpentinites where high Mg in pore solutions apparently inhibits calcite growth and promotes aragonite precipitation.
Aragonite is metastable at the low pressures near the Earth's surface and is thus commonly replaced by calcite in fossils. Aragonite older than the Carboniferous is essentially unknown.[5] It can also be synthesized by adding a calcium chloride solution to a sodium carbonate solution at temperatures above 60 °C (140 °F) or in water-ethanol mixtures at ambient temperatures.[6]
Physical properties
Aragonite is thermodynamically unstable at standard temperature and pressure, and tends to alter to calcite on scales of 107 to 108 years. The mineral vaterite, also known as μ-CaCO3, is another phase of calcium carbonate that is metastable at ambient conditions typical of Earth's surface, and decomposes even more readily than aragonite.
Uses
In aquaria, aragonite is considered essential for the replication of reef conditions. Aragonite provides the materials necessary for much sea life and also keeps the pH of the water close to its natural level, to prevent the dissolution of biogenic calcium carbonate.[7]
Aragonite has been successfully tested for the removal of pollutants like zinc, cobalt and lead from contaminated wastewaters.[8]
Gallery
 Aragonite crystals from Cuenca, Castile-La Mancha, Spain
Aragonite crystals from Cuenca, Castile-La Mancha, Spain Cluster of twinned aragonite from Morocco
Cluster of twinned aragonite from Morocco Remnant biogenic aragonite (thin, rainbow-colored shell) on the ammonite Baculites (Pierre Shale, Late Cretaceous, South Dakota).
Remnant biogenic aragonite (thin, rainbow-colored shell) on the ammonite Baculites (Pierre Shale, Late Cretaceous, South Dakota). Scanning electron microscope image of aragonite layers in the nacre of a blue mussel (Mytilus edulis).
Scanning electron microscope image of aragonite layers in the nacre of a blue mussel (Mytilus edulis). Pink Aragonite Crystals
Pink Aragonite Crystals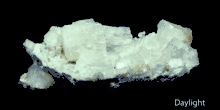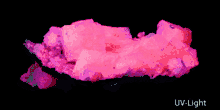 Fluorescence of Aragonite
Fluorescence of Aragonite
See also
- Aragonite sea
- Ikaite, CaCO3·6H2O
- Monohydrocalcite, CaCO3·H2O
- Nacre, otherwise known as "Mother-of-Pearl"
- Oolitic aragonite sand
References
- Mindat.org
- Handbook of Mineralogy
- Webmineral data
- Calvo, Miguel (2012). Minerales y Minas de España. Vol. V. Carbonatos y Nitratos. Madrid: Escuela Técnica Superior de Ingenieros de Minas de Madrid. Fundación Gómez Pardo. pp. 314–398. ISBN 978-84-95063-98-4.
- Runnegar, B. (1987). "Shell microstructures of Cambrian molluscs replicated by phosphate". Alcheringa: An Australasian Journal of Palaeontology. 9 (4): 245–257. doi:10.1080/03115518508618971.
- Sand, K.K., Rodriguez-Blanco, J.D., Makovicky, E., Benning, L.G. and Stipp, S. (2012) Crystallization of CaCO3 in water-ethanol mixtures: spherulitic growth, polymorph stabilization and morphology change. Crystal Growth and Design, 12, 842-853. doi:10.1021/cg2012342.
- Orr, J. C., et al. (2005) Anthropogenic ocean acidification over the 21st century and its impact on calcifying organisms. Nature 437: 681-686
- Köhler, S., Cubillas, et al. (2007) Removal of cadmium from wastewaters by aragonite shells and the influence of other divalent cations. Environmental Science and Technology, 41, 112-118. doi:10.1021/es060756j