Syringic acid
Syringic acid is a naturally occurring phenolic compound and dimethoxybenzene that is commonly found as a plant metabolite.
 | |
| Names | |
|---|---|
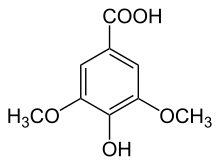
| IUPAC name
4-Hydroxy-3,5-dimethoxybenzoic acid | |
| Other names
Gallic acid 3,5-dimethyl ether | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChEMBL | |
| ECHA InfoCard | 100.007.716 |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| Properties | |
| C9H10O5 | |
| Molar mass | 198.174 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Natural occurrence
Syringic acid can be found in several plants including Ardisia elliptica and Schumannianthus dichotomus.[1]
Synthesis
Syringic acid can be prepared by selectively hydrolyzing (demethylating) eudesmic acid with 20% sulfuric acid.[2]
Presence in food
Syringic acid can be found in several fruits including olives, dates, spices, pumpkin, grapes,[3] acai palm,[4] honey, red wine, among others.[5] Its presence in the ancient Egyptian drink shedeh could confirm it was made out of grape, as syringic acid is released by the breakdown of the compound malvidin, also found in red wine. It is also found in vinegar.[6]
Applications
Various studies have found syringic acid to exhibit useful pharmaceutical properties such as anti-oxidant, anti-microbial, anti-inflammation, anti-cancer, and anti-diabetic.[5]
Syringic acid can be enzymatically polymerised. Laccase and peroxidase induced the polymerization of syringic acid to give a poly(1,4-phenylene oxide) bearing a carboxylic acid at one end and a phenolic hydroxyl group at the other.[7]
See also
References
- Rob, Md. Mahfuzur; Hossen, Kawsar; Iwasaki, Arihiro; Suenaga, Kiyotake; Kato-Noguchi, Hisashi (2020-01-14). "Phytotoxic Activity and Identification of Phytotoxic Substances from Schumannianthus dichotomus". Plants. 9 (1): 102. doi:10.3390/plants9010102. ISSN 2223-7747. PMC 7020185. PMID 31947649.
- Bogert, Marston; Ehrlich, Jacob (Mar 1919). "The synthesis of certain pyrogallol ethers, including a new acetophenetide derived from the ethyl ether of syringic acid". Journal of the American Chemical Society. 41 (5): 798–810. doi:10.1021/ja02226a013. Retrieved 2 November 2013.
- Pezzuto, John M. (August 2008). "Grapes and Human Health: A Perspective". Journal of Agricultural and Food Chemistry. 56 (16): 6777–6784. doi:10.1021/jf800898p. ISSN 0021-8561. PMID 18662007.
- Pacheco-Palencia LA, Mertens-Talcott S, Talcott ST (Jun 2008). "Chemical composition, antioxidant properties, and thermal stability of a phytochemical enriched oil from Acai (Euterpe oleracea Mart.)". J Agric Food Chem. 56 (12): 4631–4636. doi:10.1021/jf800161u. PMID 18522407.CS1 maint: uses authors parameter (link)
- Srinivasulu, Cheemanapalli; Ramgopal, Mopuri; Ramanjaneyulu, Golla; Anuradha, C.M.; Suresh Kumar, Chitta (December 2018). "Syringic acid (SA) ‒ A Review of Its Occurrence, Biosynthesis, Pharmacological and Industrial Importance". Biomedicine & Pharmacotherapy. 108: 547–557. doi:10.1016/j.biopha.2018.09.069. ISSN 0753-3322. PMID 30243088.
- Analysis of polyphenolic compounds of different vinegar samples. Miguel Carrero Gálvez, Carmelo García Barroso and Juan Antonio Pérez-Bustamante, Zeitschrift für Lebensmitteluntersuhung und -Forschung A, Volume 199, Number 1, pages 29–31, doi:10.1007/BF01192948
- Uyama, Hiroshi; Ikeda, Ryohei; Yaguchi, Shigeru; Kobayashi, Shiro (2001). "Enzymatic Polymerization of Natural Phenol Derivatives and Enzymatic Synthesis of Polyesters from Vinyl Esters". Polymers from Renewable Resources. ACS Symposium Series. 764. p. 113. doi:10.1021/bk-2000-0764.ch009. ISBN 0-8412-3646-1.