Ethyl gallate
Ethyl gallate is a food additive with E number E313. It is the ethyl ester of gallic acid. Ethyl gallate is added to food as an antioxidant.
 | |
 | |
| Names | |
|---|---|
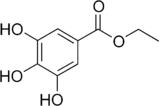
| IUPAC name
Ethyl 3,4,5-trihydroxybenzoate | |
| Other names
Phyllemblin gallic acid ethyl ester | |
| Identifiers | |
3D model (JSmol) |
|
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.011.462 |
| E number | E313 (antioxidants, ...) |
PubChem CID |
|
| RTECS number |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C9H10O5 | |
| Molar mass | 198.17 g/mol |
| Melting point | 149 to 153 °C (300 to 307 °F; 422 to 426 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Though found naturally in a variety of plant sources including walnuts[2] Terminalia myriocarpa[3] or chebulic myrobolan (Terminalia chebula)[4], ethyl gallate is produced from gallic acid and ethanol.[5] It can be found in wine.[6]
See also
References
- Ethyl gallate at Sigma-Aldrich
- Zijia Zhanga; Liping Liaoc; Jeffrey Moored; Tao Wua; Zhengtao Wanga (2009). "Antioxidant phenolic compounds from walnut kernels (Juglans regia L.)". Food Chemistry. 113 (1): 160–165. doi:10.1016/j.foodchem.2008.07.061.
- Pharmacologically Active Ellagitannins from Terminalia myriocarpa. Mohamed S.A. Marzouk, Sayed A.A. El-Toumy, Fatma A. Moharram, Nagwa M.M. Shalaby and Amany A.E. Ahmed, Planta Med, 2002, 68(6), pages 523-527, doi:10.1055/s-2002-32549
- "Haritaki". Toddcaldecott.com. Archived from the original on 2013-12-03. Retrieved 2014-05-18.
- Enzymic synthesis of gallic acid esters. Weetall, Howard Hayyim. Eur. Pat. 137601 (1985)
- Bartolomé, Begoña; Gómez-Cordovés, Carmen; Suárez, Rafael; Monagas, María (2005-06-01). "Simultaneous Determination of Nonanthocyanin Phenolic Compounds in Red Wines by HPLC-DAD/ESI-MS. María Monagas, Rafael Suárez, Carmen Gómez-Cordovés and Begoña Bartolomé, AJEV, June 2005, vol. 56, no. 2, pages 139-147". American Journal of Enology and Viticulture. 56 (2): 139–147. Retrieved 2014-05-18.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.