Sulfur dye
Sulfur dyes are the most commonly used dyes manufactured for cotton in terms of volume. They are inexpensive, generally have good wash-fastness, and are easy to apply. Sulfur dyes are predominantly black, brown, and dark blue.[1] Red sulfur dyes are unknown, although a pink or lighter scarlet color is available.
Chemistry
Sulphur linkages are the integral part of chromophore in sulfur dyes. They are organosulfur compounds consisting of sulphide (–S–), disulphide (–S–S–) and polysulphide (–Sn–) links in heterocyclic rings. They feature thiazoles, thiazone, thianthrene, and phenothiazonethioanthrone subunits. Being nonionic, sulphur dyes are insoluble in water.
Process
Dyeing includes a few stages, viz. reduction, dyeing, washing, oxidation, soaping and final washing. The anion is developed on reducing and solubilising at boil when it shows affinity for cellulose. Sodium sulphide (Na2S), the reducing cum solubilising agent perform both reduction and solubilisation producing thiols and then to sodium salt of thiols or thiolates, which are soluble in water and substantive towards cellulose. Higher rate of exhaustion occurs at 90-95oC in presence of electrolyte. Dyed cellulosics exhibit tendering effect on storage under humid atmosphere due to presence of excess free sulphur and aftertreatment with sodium acetate is required to suppress that. H2S liberated during dyeing forms corrosive metal sulphide and this restricts use of metal vessels except those made of stainless steel. Fe + H2S → FeS + H2[2]
Production, past and present
The forerunner of sulfur dyes is attributed to "Cachou de Laval," which is prepared by treating wood products with sulfide sources. Subsequently, the so-called Vidal Blacks were produced by reactions of various aniline derivatives with sulfur. These experiments demonstrated that deeply colored materials could be readily produced by combining aromatic compounds and sulfur sources.[3]
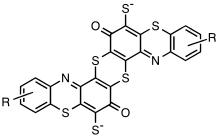
The most important member of the class is Sulfur Black 1. It is produced by the reaction of 2,4-dinitrophenol and sodium sulfide in hot water. Like many sulfur dyes, details on the chemical reactions are poorly understood. It is accepted that the sulfide reduces the nitro groups to aniline derivatives, which are thought to form indophenol-containing intermediates that are further crosslinked by reaction with sulfur. The result are insoluble, high molecular weight species. Sulfur Black 1 is imperfectly understood, and the material is probably heterogeneous. It is speculated to be a polymer consisting of thianthrene and phenothiazine subunits. The so-called sulfur bake dyes are produced from 1,4-diaminobenzene and diaminotoluene derivatives. These dyes are proposed to consist of polymers with benzothiazole subunits. Members of the sulfur bake dyes class are Sulfur Orange 1, Sulfur Brown 21, and Sulfur Green 12.[1]
Application method
Sulfur dyes are water-insoluble. In the presence of a reducing agent and at alkali pH's at elevated temperature of around 80 °C, the dye particles disintegrate, which then becomes water-soluble and hence can be absorbed by the fabric. Sodium sulfide or sodium hydrosulfide are suitable reducing agents. Common salt facilitates the absorption. After the fabric is removed from the dye solution, it is allowed to stand in air whereupon the dye is regenerated by oxidation. The regenerated parent dye is insoluble in water. Oxidation can also be effected in air or by hydrogen peroxide or sodium bromate in a mildly acidic solution.
The low water solubility is the basis of the good wash-fastness of these dyed fabrics. These dyes have good all round fastness except to chlorine bleaches. Because the dye is water-insoluble, it will not bleed when washed in water and will not stain other clothes. The dye, however, may have poor fastness to rubbing. The dyes are bleached by hypochlorite bleach.
Environmental issues
Due to the highly polluting nature of the dye-bath effluent, sulfur dyes are being slowly phased out in the West but they are used on a large scale in China.[3] Recent advances in dyeing technologies have allowed the substitution of toxic sulfide reducing agents. Glucose in basic solution is now used and both low sulfide and zero sulfide products are available. Future developments in the field of reducing dye levels by means of electro-chemical processes are promising.
References
- Nagl, Gert (2000). "Sulfur Dyes". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a25_613.
- Peters R. H, “Textile Chemistry”, Vol - II, Elsevier Publishing Company, London (1967)
- Parikshit Goswami, Montu Basak "Sulfur Dyes" in Kirk-Othmer Encyclopedia of Chemical Technology, 2001, John Wiley & Sons. doi:10.1002/0471238961.1921120619051409.a01.pub2.
- Industrial Dyes: Chemistry, Properties, Applications" Klaus Hunger, Ed. 2007, Wiley-VCH, Weinheim. ISBN 3-527-30426-6
