Polyketide
Polyketides are a large group of secondary metabolites which either contain alternating carbonyl groups and methylene groups (-CO-CH2-), or are derived from precursors which contain such alternating groups. [1]
Many polyketides have antimicrobial and immunosuppressive properties. Many mycotoxins produced by fungi are polyketides.[2]
- Polyketides
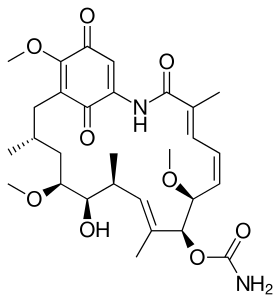 Geldanamycin, a useful antibiotic.
Geldanamycin, a useful antibiotic.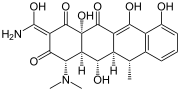 Doxycycline, another important antibiotic.
Doxycycline, another important antibiotic.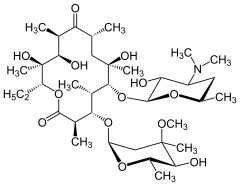 Erythromycin, an antibiotic.
Erythromycin, an antibiotic.-Aflatoxin_B1_Structural_Formulae_V.1.svg.png) Aflatoxin B1 one of the most carcinogenic compounds known.
Aflatoxin B1 one of the most carcinogenic compounds known.
Biosynthesis
Polyketides are synthesized in bacteria, fungi, plants, and certain marine animals by the stepwise condensation of acetyl-CoA or propionyl-CoA with malonyl-CoA or methylmalonyl-CoA extender units. Each condensation reaction is driven by the decarboxylation of the extender unit and yields a beta-keto functional group (e.g., an acetoacetyl group; this is why the ultimate product is called a polyketide). [3][4]

The polyketide chains produced by a minimal polyketide synthase are often further derivatized and modified into bioactive natural products. [5]
Palmitic acid is an example of a polyketide since it is formed by the condensation of one acetyl-CoA primer and seven malonyl-CoA extender units. Following each condensation reaction, the new beta-keto group may be reduced, dehydrated, and reduced again as with fatty acids and therefore the final product may not contain any actual ketide subunits itself.[3]
Polyketides are synthesized by multienzyme polypeptides that resemble eukaryotic fatty acid synthase but are often much larger. They include acyl-carrier domains plus an assortment of enzymatic units that can function in an iterative fashion, repeating the same elongation/modification steps (as in fatty acid synthesis), or in a sequential fashion so as to generate more heterogeneous types of polyketides.[3]
Polyketides are structurally a very diverse family of natural products with diverse biological activities and pharmacological properties.[6] They are broadly divided into three classes: type I polyketides (often macrolides produced by multimodular megasynthases), type II polyketides (often aromatic molecules produced by the iterative action of dissociated enzymes), and type III polyketides (often small aromatic molecules produced by fungal species). Polyketide antibiotics, antifungals, cytostatics, anticholesteremic, antiparasitics, coccidiostats, animal growth promoters and natural insecticides are in commercial use.
Examples
- Macrolides
- Pikromycin, the first isolated macrolide (1951[7])
- The antibiotics erythromycin A, clarithromycin, and azithromycin
- The antihelminthics avermectin and ivermectin
- The insecticide spinosad (spinosyn)
- Ansamycins
- The antitumor agents geldanamycin and macbecin,
- The antibiotic rifamycin
- Polyenes
- The antifungals amphotericin, nystatin and pimaricin
- Polyethers
- The antibiotic monensin
- Tetracyclines
- The antibiotic agent doxycycline
- Acetogenins
- bullatacin
- squamocin
- molvizarin
- uvaricin
- annonacin
- Others
- The immunosuppressants tacrolimus (FK506) (a calcineurin inhibitor) and sirolimus (rapamycin) (a mTOR inhibitor)
- Radicicol and the pochonin family (HSP90 inhibitors)
- The cholesterol lowering agent lovastatin
- Discodermolide
- Aflatoxin
- Usnic acid
- Anthracimycin
- Anthramycin
References
- IUPAC, Compendium of Chemical Terminology, 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) "Polyketides". doi:10.1351/goldbook.P04734
- Huffman, Justin; Gerber, Ryan; Du, Liangcheng (2010). "Recent advancements in the biosynthetic mechanisms for polyketide-derived mycotoxins". Biopolymers. 93 (9): 764–776. doi:10.1002/bip.21483. PMC 2894268. PMID 20578001.
- Voet, Donald; Voet, Judith G.; Pratt, Charlotte W. (2013). Fundamentals of Biochemistry: Life at the Molecular Level (4th ed.). John Wiley & Sons. p. 688. ISBN 9780470547847.
- Staunton, James; Weissman, Kira J. (2001). "Polyketide Biosynthesis: A Millennium Review". Natural Product Reports. 18 (4): 380–416. doi:10.1039/a909079g.
- Robinson, John A.; Fersht, Alan Roy; Gani, D. (1991). "Polyketide synthase complexes: Their structure and function in antibiotic biosynthesis". Philos. Trans. R. Soc. Lond. B Biol. Sci. 332 (1263): 107–114. doi:10.1098/rstb.1991.0038. PMID 1678529.
- Katz, Leonard (1997). "Manipulation of Modular Polyketide Synthases". Chem. Rev. 97 (7): 2557–2576. doi:10.1021/cr960025+.
- Brockmann, Hans; Henkel, Willfried (1951). "Pikromycin, ein bitter schmeckendes Antibioticum aus Actinomyceten" [Pikromycin, a bitter tasting antibiotic from an actinomycete]. Chem. Ber. (in German). 84 (3): 284–288. doi:10.1002/cber.19510840306.