Protein phosphatase 1
Protein phosphatase 1 (PP1) belongs to a certain class of phosphatases known as protein serine/threonine phosphatases. This type of phosphatase includes metal-dependent protein phosphatases (PPMs) and aspartate-based phosphatases. PP1 has been found to be important in the control of glycogen metabolism, muscle contraction, cell progression, neuronal activities, splicing of RNA, mitosis,[1] cell division, apoptosis, protein synthesis, and regulation of membrane receptors and channels.[2]
Structure
Each PP1 enzyme contains both a catalytic subunit and at least one regulatory subunit.[3][4] The catalytic subunit consists of a 30-kD single-domain protein that can form complexes with other regulatory subunits. The catalytic subunit is highly conserved among all eukaryotes, thus suggesting a common catalytic mechanism. The catalytic subunit can form complexes with various regulatory subunits. These regulatory subunits play an important role in substrate specificity as well as compartmentalization. Some common regulatory subunits include GM (PPP1R3A) and GL (PPP1R3B), which are named after their locations of action within the body (Muscle and Liver respectively).[5] While the yeast S. cerevisiae only encodes one catalytic subunit, mammals have four isozymes encoded by three genes, each attracting a different set of regulatory subunits.[4]
X-ray crystallographic structural data is available for PP1 catalytic subunit.[3] The catalytic subunit of PP1 forms an α/β fold with a central β-sandwich arranged between two α-helical domains. The interaction of the three β-sheets of the β-sandwich creates a channel for catalytic activity, as it is the site of coordination of metal ions.[6] These metal ions have been identified as Mn and Fe and their coordination is provided by three histidines, two aspartic acids, and one asparagine.[7]
Mechanism
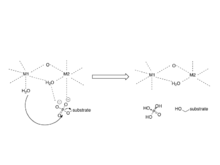
The mechanism involves two metal ions binding and activating water, which initiates a nucleophilic attack on the phosphorus atom.[8]
Regulation
Regulation of these different processes is performed by distinct PP1 holoenzymes that facilitate the complexation of the PP1 catalytic subunit to various regulatory subunits.[4]
Potential inhibitors include a variety of naturally occurring toxins including okadaic acid, a diarrhetic shellfish poison, strong tumor promoter, and microcystin.[9] Microcystin is a liver toxin produced by blue-green algae and contains a cyclic heptapeptide structure that interacts with three distinct regions of the surface of the catalytic subunit of PP1.[10] The structure of MCLR does not change when complexed with PP1, but the catalytic subunit of PP1 does in order to avoid steric effects of Tyr 276 of PP1 and Mdha side chain of MCLR.[7]
Biological function
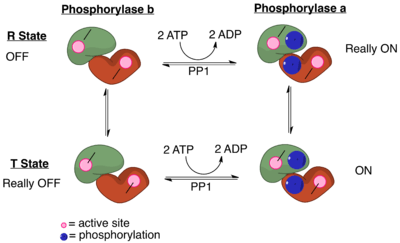
PP1 plays a crucial role in the regulation of blood-glucose levels in the liver and glycogen metabolism. PP1 is important to the reciprocal regulation of glycogen metabolism by ensuring the opposite regulation of glycogen breakdown and glycogen synthesis. Phosphorylase a serves as a glucose sensor in liver cells.[11] When glucose levels are low, phosphorylase a in its active R state has PP1 bound tightly. This binding to phosphorylase a prevents any phosphatase activity of PP1 and maintains the glycogen phosphorylase in its active phosphorylated configuration. Therefore, there phosphorylase a will accelerate glycogen breakdown until adequate levels of glucose are achieved.[11] When glucose concentrations get too high, phosphorylase a is converted to its inactive, T state. By shifting phosphorylase a to its T state, PP1 dissociates from the complex. This dissociation activates glycogen synthase and converts phosphorylase a to phosphorylase b. Phosphorylase b does not bind PP1 allowing PP1 to remain activated.[11]
When the muscles of the body signal for the need for glycogen degradation and increased glucose concentration, PP1 will be regulated accordingly. Protein kinase A can reduce the activity of PP1. The glycogen binding region, GM, becomes phosphorylated, which causes its dissociation from the catalytic PP1 unit.[11] This separation of the catalytic PP1 unit, glycogen, and other substrates causes a significant decrease in dephosphorylation. Also, when other substrates become phosphorylated by protein kinase A, they can bind to the catalytic subunit of PP1 and directly inhibit it.[11] In the end, phosphorylase is kept in its active form and glycogen synthase in its inactive form.
Disease relevance
In Alzheimer’s, hyperphosphorylation of the microtubule-associated protein inhibits the assembly of microtubules in neurons. Researchers at the New York State Institute for Basic Research in Developmental Disabilities showed that there is significantly lower type 1 phosphatase activity in both gray and white matters in Alzheimer disease brains.[12] This suggests that dysfunctional phosphatases play a role in Alzheimer's disease.
Regulation of HIV-1 transcription by Protein Phosphatase 1 (PP1). It has been recognized that protein phosphatase-1 (PP1) serves as an important regulator of HIV-1 transcription. Researchers at Howard University showed that Tat protein targets PP1 to the nucleus and the consequent interaction is important for HIV-1 transcription.[13] The protein also contributes to ebolavirus pathogenesis by dephosphorylating the viral transcription activator VP30, allowing it to produce viral mRNAs. Inhibition of PP1 prevents VP30 dephosphorylation, thus preventing manufacture of viral mRNA, and thus viral protein. The viral L polymerase is, however, still capable of replicating viral genomes without VP30 dephosphorylation by PP1.[14]
The herpes simplex virus protein ICP34.5 also activates protein phosphatase 1, which overcomes the cellular stress response to viral infection; protein kinase R is activated by the virus' double-stranded RNA, and protein kinase R then phosphorylates a protein called eukaryotic initiation factor-2A (eIF-2A), which inactivates eIF-2A. EIF-2A is required for translation so by shutting down eIF-2A, the cell prevents the virus from hijacking its own protein-making machinery. Herpesviruses in turn evolved ICP34.5 to defeat the defense; ICP34.5 activates protein phosphatase-1A which dephosphorylates eIF-2A, allowing translation to occur again. ICP34.5 shares the C-terminal regulatory domain (InterPro: IPR019523) with protein phosphatase 1 subunit 15A/B.[15]
Subunits
| protein phosphatase 1, catalytic subunit, alpha isozyme | |
|---|---|
| Identifiers | |
| Symbol | PPP1CA |
| Alt. symbols | PP1, PP1a, MGC15877, MGC1674, PP-1A, PP1alpha, PPP1A |
| NCBI gene | 5499 |
| HGNC | 9281 |
| OMIM | 176875 |
| RefSeq | NP_002699.1 |
| UniProt | P62136 |
| Other data | |
| EC number | 3.1.3.16 |
| Locus | Chr. 11 q13 |
| protein phosphatase 1, catalytic subunit, beta isozyme | |
|---|---|
| Identifiers | |
| Symbol | PPP1CB |
| Alt. symbols | PP1, PP1b, PP1beta, PP-1B; PPP1CD; MGC3672; PP1beta; PPP1CB |
| NCBI gene | 5500 |
| HGNC | 9282 |
| OMIM | 600590 |
| RefSeq | NP_002700.1 |
| UniProt | P62140 |
| Other data | |
| EC number | 3.1.3.16 |
| Locus | Chr. 2 p23 |
| protein phosphatase 1, catalytic subunit, gamma isozyme | |
|---|---|
| Identifiers | |
| Symbol | PPP1CC |
| Alt. symbols | PP1gamma, PP1y, PP1gamma, PPP1G |
| NCBI gene | 5501 |
| HGNC | 9283 |
| OMIM | 176914 |
| RefSeq | NP_002701.1 |
| UniProt | P36873 |
| Other data | |
| EC number | 3.1.3.16 |
| Locus | Chr. 12 q24 |
Protein phosphatase 1 is a multimeric enzyme that may contain the following subunits:[16]
- catalytic subunit: PPP1CA, PPP1CB, PPP1CC
- regulatory subunit 1: PPP1R1A, PPP1R1B, PPP1R1C
- regulatory subunit 2: PPP1R2
- regulatory subunit 3: PPP1R3A, PPP1R3B, PPP1R3C, PPP1R3D, PPP1R3E, PPP1R3F, PPP1R3G
- regulatory subunit 7: PPP1R7
- regulatory subunit 8: PPP1R8
- regulatory subunit 9: PPP1R9A, PPP1R9B
- regulatory subunit 10: PPP1R10
- regulatory subunit 11: PPP1R11
- regulatory subunit 12: PPP1R12A, PPP1R12B, PPP1R12C
- regulatory subunit 13: PPP1R13B
- regulatory subunit 14: PPP1R14A, PPP1R14B, PPP1R14C, PPP1R14D
- regulatory subunit 15: PPP1R15A, PPP1R15B
- regulatory subunit 16: PPP1R16A, PPP1R16B
As described earlier, a catalytic subunit is always paired with one or more regulatory subunits. The core sequence motif for binding to the catalytic subunit is "RVxF", but additional motifs allow for extra sites to be used. Some complexes with two regulatory subunits attached have been reported in 2002 and 2007.[4]
References
- Tournebize R, Andersen SS, Verde F, Dorée M, Karsenti E, Hyman AA (September 1997). "Distinct roles of PP1 and PP2A-like phosphatases in control of microtubule dynamics during mitosis". The EMBO Journal. 16 (18): 5537–49. doi:10.1093/emboj/16.18.5537. PMC 1170186. PMID 9312013.
- Fong NM, Jensen TC, Shah AS, Parekh NN, Saltiel AR, Brady MJ (November 2000). "Identification of binding sites on protein targeting to glycogen for enzymes of glycogen metabolism". The Journal of Biological Chemistry. 275 (45): 35034–9. doi:10.1074/jbc.M005541200. PMID 10938087.
- Goldberg J, Huang HB, Kwon YG, Greengard P, Nairn AC, Kuriyan J (August 1995). "Three-dimensional structure of the catalytic subunit of protein serine/threonine phosphatase-1". Nature. 376 (6543): 745–53. Bibcode:1995Natur.376..745G. doi:10.1038/376745a0. PMID 7651533.
- Virshup DM, Shenolikar S (March 2009). "From promiscuity to precision: protein phosphatases get a makeover". Molecular Cell. 33 (5): 537–45. doi:10.1016/j.molcel.2009.02.015. PMID 19285938.
- Armstrong CG, Browne GJ, Cohen P, Cohen PT (November 1997). "PPP1R6, a novel member of the family of glycogen-targetting subunits of protein phosphatase 1". FEBS Letters. 418 (1–2): 210–4. doi:10.1016/S0014-5793(97)01385-9. PMID 9414128.
- Egloff MP, Johnson DF, Moorhead G, Cohen PT, Cohen P, Barford D (April 1997). "Structural basis for the recognition of regulatory subunits by the catalytic subunit of protein phosphatase 1". The EMBO Journal. 16 (8): 1876–87. doi:10.1093/emboj/16.8.1876. PMC 1169791. PMID 9155014.
- Barford D, Das AK, Egloff MP (1998). "The structure and mechanism of protein phosphatases: insights into catalysis and regulation". Annual Review of Biophysics and Biomolecular Structure. 27: 133–64. doi:10.1146/annurev.biophys.27.1.133. PMID 9646865.
- Zhang Y, Zhang M, Zhang Y (March 2011). "Crystal structure of Ssu72, an essential eukaryotic phosphatase specific for the C-terminal domain of RNA polymerase II, in complex with a transition state analogue". The Biochemical Journal. 434 (3): 435–44. doi:10.1042/BJ20101471. PMID 21204787.
- Wera S, Hemmings BA (October 1995). "Serine/threonine protein phosphatases". The Biochemical Journal. 311 ( Pt 1) (1): 17–29. doi:10.1042/bj3110017. PMC 1136113. PMID 7575450.
- MacKintosh C, Beattie KA, Klumpp S, Cohen P, Codd GA (May 1990). "Cyanobacterial microcystin-LR is a potent and specific inhibitor of protein phosphatases 1 and 2A from both mammals and higher plants". FEBS Letters. 264 (2): 187–92. doi:10.1016/0014-5793(90)80245-E. PMID 2162782.
- Berg JM, Stryer L, Tymoczko JL (2010-12-24). Biochemistry (7th ed.). New York: W.H. Freeman. ISBN 978-1-4292-2936-4.
- Gong CX, Singh TJ, Grundke-Iqbal I, Iqbal K (September 1993). "Phosphoprotein phosphatase activities in Alzheimer disease brain". Journal of Neurochemistry. 61 (3): 921–7. doi:10.1111/j.1471-4159.1993.tb03603.x. PMID 8395566.
- Nekhai S, Jerebtsova M, Jackson A, Southerland W (January 2007). "Regulation of HIV-1 transcription by protein phosphatase 1". Current HIV Research. 5 (1): 3–9. doi:10.2174/157016207779316279. PMID 17266553.
- Ilinykh PA, Tigabu B, Ivanov A, Ammosova T, Obukhov Y, Garron T, et al. (August 2014). "Role of protein phosphatase 1 in dephosphorylation of Ebola virus VP30 protein and its targeting for the inhibition of viral transcription". The Journal of Biological Chemistry. 289 (33): 22723–38. doi:10.1074/jbc.M114.575050. PMC 4132779. PMID 24936058.
- Agarwalla PK, Aghi MK (2012). "Oncolytic herpes simplex virus engineering and preparation". Methods in Molecular Biology. 797: 1–19. doi:10.1007/978-1-61779-340-0_1. ISBN 978-1-61779-339-4. PMID 21948465.
- Cohen PT (January 2002). "Protein phosphatase 1--targeted in many directions". Journal of Cell Science. 115 (Pt 2): 241–56. PMID 11839776.
External links
- Protein+Phosphatase+1 at the US National Library of Medicine Medical Subject Headings (MeSH)