Post-metallocene catalyst
A post-metallocene catalyst is a kind of catalyst for the polymerization of olefins, i.e., the industrial production of some of the most common plastics. "Post-metallocene" refers to a class of homogeneous catalysts that are not metallocenes. This area has attracted much attention because the market for polyethylene, polypropylene, and related copolymers is large. There is a corresponding intense market for new processes as indicated by the fact that, in the US alone, 50,000 patents were issued between 1991-2007 on polyethylene and polypropylene.[1]
Many methods exist to polymerize alkenes, including the traditional routes using Philips catalyst and traditional heterogeneous Ziegler-Natta catalysts, which still are used to produce the bulk of polyethylene.
Catalysts based on early transition metals
- Early metal post-metallocene catalyst designs
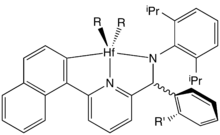 Generic structure of a post-metallocene catalyst based on Dow's pyridyl-amido design.
Generic structure of a post-metallocene catalyst based on Dow's pyridyl-amido design.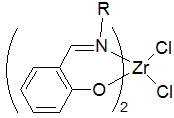 Early examples of postmetallocene catalysts included Schiff base ligands.
Early examples of postmetallocene catalysts included Schiff base ligands.
Homogeneous metallocene catalysts, e.g., derived from or related to zirconocene dichloride introduced a level of microstructural control that was unavailable with heterogeneous systems.[2] Metallocene catalysts are homogeneous single-site systems, implying that a uniform catalyst is present in the solution. In contrast, commercially important Ziegler-Natta heterogeneous catalysts contain a distribution of catalytic sites. The catalytic properties of single-site catalysts can be controlled by modification of the ligand. Initially ligand modifications focused on various cyclopentadienyl derivatives, but great diversity was uncovered through high throughput screening. These post-metallocene catalysts employ a range of chelating ligands, often including pyridine and amido (R2N−). These ligands are available in great diversity with respect to their steric and electronic properties. Such postmetallocene catalysts enabled the introduction of Chain shuttling polymerization.[1]
Catalysts based on late transition metals
The copolymerization of ethylene with polar monomers has been heavily studied. The high oxophilicity of the early metals precluded their use in this application.[3]
- Late metal post-metallocene catalyst designs
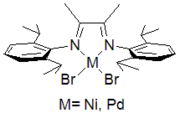 Catalyst supported by charge-neutral alpha-diimine ligands.
Catalyst supported by charge-neutral alpha-diimine ligands.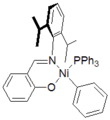 Catalyst supported by anionic Schiff base ligand
Catalyst supported by anionic Schiff base ligand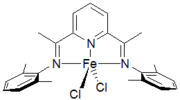 Catalysts supported by tridentate diiminopyridine ligand.
Catalysts supported by tridentate diiminopyridine ligand.
Efforts to copolymerize polar comonomers led to interest in catalysts based upon nickel and palladium, inspired by the success of the Shell Higher Olefin Process. Typical post-metallocene catalysts feature bulky, neutral, alpha-diimine ligands.[3] The technology was further developed in the laboratories of DuPont’s Central Research. They have been commercialized as DuPont’s Versipol olefin polymerization system.[4] Eastman commercialized the related Gavilan technology.[5] These complexes catalyze the homopolymerize ethylene to a variety of structures that range from high density polyethylene through hydrocarbon plastomers and elastomers by a mechanism referred to as “chain-walking”. By reducing the bulk of the alpha-diimine used, the product distribution of these systems can be 'tuned' to consist of hydrocarbon oils (alpha-olefins), similar to those produced by more tradition nickel(II) oligo/polymerization catalysts. As opposed to metallocenes, they can also randomly copolymerize ethylene with polar comonomers such as methyl acrylate.
A second class of complexes were discovered simultaneously by Robert H. Grubbs[6] and DuPont.[7] These catalysts feature mono-anionic bidentate ligands related to salen ligands. One interesting feature of these catalysts was that pendant Lewis acid functionality could be incorporated to direct the incorporation of polar comonomers. These catalysts are often better suited for the preparation of specialty oligomers and polymers incorporating reactive functionality.
The concept of bulky bis-imine ligands was extended to iron complexes[3] Representative catalysts feature diiminopyridine ligands. These catalysts, illustrated to the left, are highly active do not promote chain walking. As a result, these complexes give very linear high-density polyethylene when bulky and when the steric bulk is removed, they are very active catalysts for ethylene oligomerization to linear alpha-olefins.[3]
A salicylimine catalyst system based on zirconium, shown to the right, exhibits high activity for ethylene polymerization.[8] The catalysts can also produce some novel polypropylene structures.[9] Despite intensive efforts, few catalysts have been successfully commercialized for the copolymerization of polar monomers.
References
- Chum, P. S.; Swogger, K. W., "Olefin Polymer Technologies-History and Recent Progress at the Dow Chemical Company", Progress in Polymer Science 2008, volume 33, 797-819. doi:10.1016/j.progpolymsci.2008.05.003
- Brintzinger, H. H.; Fischer, D.; Muelhaupt, R.; Rieger, B.; Waymouth, R. M., "Stereospecific Olefin Polymerization with Chiral Metallocene Catalysts", Angew. Chem. Int. Ed. Engl. 1995, 34, 1143-1170. doi:10.1002/anie.199511431
- Domski, G. J., Rose, J. M., Coates, G. W., Bolig, A. D., Brookhart, M., "Living alkene polymerization: New methods for the precision synthesis of polyolefins", Prog. Polymer Sci. 2007, volume 32, p.30. doi:10.1016/j.progpolymsci.2006.11.001
- US 5,866,663 "Process of Polymerizing Olefins," Samuel David Arthur, Alison Margaret Anne Bennett, Maurice S. Brookhart, Edward Bryan Coughlin, Jerald Feldman, Steven Dale Ittel, Lynda Kaye Johnson, Christopher Moore Killian; Kristina Ann Kreutzer, Elizabeth Forrester McCord, Stephan James McLain, Anju Parthasarathy, Lin Wang, Zhen-Yu Yang; February 2, 1999. WO 9623010 A2 960801.
- MacKenzie, P. B.; Moody, L. S.; Killian, C. M.; Ponasik, J. A.; McDevitt, J. P. WO Patent Application 9840374, Sept. 17, 1998 to Eastman, priority date Feb 24, 1998.
- C. Wang, S. Friedrich, T. R. Younkin, R. T. Li, R. H. Grubbs, D. A. Bansleben, M. W. Day, Organometallics, 17, 3149 (1998).
- US 6,174,975, “Polymerization of Olefins,” Lynda Kaye Johnson; Alison Margaret Anne Bennett, Lin Wang, Anju Parthasarathy, Elisabeth Hauptman, Robert D. Simpson, Jerald Feldman, Edward Bryan Coughlin, and Steven Dale Ittel. January 16, 2001.
- S. Matsui, Y. Tohi, M. Mitani, J. Saito, H. Makio, H. Tanaka, M. Nitabaru, T. Nakano, T, Fujita, Chem. Lett., 1065 (1999).
- Steven D. Ittel and Lynda K. Johnson and Maurice Brookhart, Late-Metal Catalysts for Ethylene Homo- and Copolymerization, Chem. Rev. 2000, 100, 1169-1203.