Diimine
Diimines are organic compounds containing two imine (RCH=NR') groups. The most popular derivatives are 1,2-diketones and 1,3-diimines. These compounds are used as ligands and as precursors to heterocycles. Diimines are prepared by condensation reactions where a dialdehyde or diketone is treated with amine and water is eliminated. Similar methods are used to prepare Schiff bases and oximes.
1,2-Diimines
The 1,2-diketimine ligands, also called α-diimines. They are derived from the condensation of 1,2-diketones with amines, using anilines.[1]
An example is glyoxal-bis(mesitylimine), a yellow solid that is synthesized by condensation of 2,4,6-trimethylaniline and glyoxal.[2] 2,2'-Bipyridine is a 1,2-diimine.
1,2-Diketimines are “non-innocent ligands”, akin to the dithiolenes.
1,3-Diimines
For example, acetylacetone (2,4-pentanedione) and a primary alkyl- or arylamine will react, typically in acidified ethanol, to form a diketimine. 1,3-Diketimines are often referred to as HNacNac, a modification of the abbreviation Hacac for the conjugate acid of acetylacetone. These species form anionic bidentate anionic ligands.
Uses
Substituted α-diimine ligands are useful in the preparation of so-called post-metallocene catalysts for the polymerization and copolymerization of ethylene and alkenes.[3]
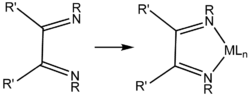
Diimines are precursors to NHC ligands by condensation with formaldehyde.[2]
References
- Wang, F.; Chen, C. (2019). "A Continuing Legend: The Brookhart-Type α-Diimine Nickel and Palladium Catalysts". Polymer Chemistry. 10: 2354-2369. doi:10.1039/C9PY00226J.
- Ison, Elon A.; Ison, Ana (2012). "Synthesis of Well-Defined Copper N-Heterocyclic Carbene Complexes and Their Use as Catalysts for a "Click Reaction": A Multistep Experiment That Emphasizes the Role of Catalysis in Green Chemistry". J. Chem. Educ. 89: 1575–1577. doi:10.1021/ed300243s.
- Ittel, S. D.; Johnson, L. K.; Brookhart, M. (2000). "Late-Metal Catalysts for Ethylene Homo- and Copolymerization". Chemical Reviews. 100: 1169–1203. doi:10.1021/cr9804644.