Pioglitazone
Pioglitazone, sold under the brand name Actos among others, is a medication used to treat type 2 diabetes.[1] It may be used with metformin, a sulfonylurea, or insulin.[1][2] Use is recommended together with exercise and diet.[2] It is not recommended in type 1 diabetes.[2] It is taken by mouth.[2]
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Actos, others |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a699016 |
| License data | |
| Pregnancy category |
|
| Routes of administration | By mouth |
| Drug class | Thiazolidinedione |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Protein binding | >99% |
| Metabolism | liver (CYP2C8) |
| Elimination half-life | 3–7 hours |
| Excretion | in bile |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.114.441 |
| Chemical and physical data | |
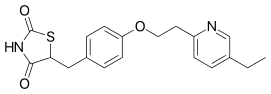
| Formula | C19H20N2O3S |
| Molar mass | 356.44 g·mol−1 |
| 3D model (JSmol) | |
| Chirality | Racemic mixture |
| Melting point | 183 to 184 °C (361 to 363 °F) |
| |
| |
| (verify) | |
Common side effects include headaches, muscle pains, inflammation of the throat, and swelling.[2] Serious side effects may include bladder cancer, low blood sugar, heart failure, and osteoporosis.[2][1] Use in not recommended in pregnancy or breastfeeding.[1] It is in the thiazolidinedione (TZD) class and works by improving sensitivity of tissues to insulin.[1]
Pioglitazone was patented in 1985, and came into medical use in 1999.[3] It is available as a generic medication.[1] A month supply in the United Kingdom costs the NHS less than £1 as of 2019.[1] In the United States the wholesale cost of this amount is about 3.20 USD.[4] In 2017, it was the 125th most commonly prescribed medication in the United States, with more than five million prescriptions.[5][6] It was withdrawn in France and Germany in 2011.[7]
Medical uses
Pioglitazone is used to lower blood glucose levels in type 2 diabetes either alone or in combination with a sulfonylurea, metformin, or insulin.[8] While pioglitazone does decrease blood sugar levels, the main study that looked at the medication found no difference in the main cardiovascular outcomes that were looked at.[9] The secondary outcome of death from all causes, myocardial infarction, and stroke were lower.[9]
Contraindications
Pioglitazone cannot be used in patients with a known hypersensitivity to pioglitazone, other thiazolidinediones or any of components of its pharmaceutical forms. It is ineffective and possibly harmful in diabetes mellitus type 1 and diabetic ketoacidosis.[8] Its safety in pregnancy, lactation (breastfeeding) and people under 18 is not established.[8]
Given previous experiences with the related drug troglitazone, acute diseases of the liver are regarded as a contraindication for pioglitazone.
Pioglitazone and all other drugs of its class (thiazolidinediones) are absolutely contraindicated in patients with heart failure.
Side effects
A press release by GlaxoSmithKline in February 2007 noted that there is a greater incidence of fractures of the upper arms, hands and feet in female diabetics given rosiglitazone compared with those given metformin or glyburide. The information was based on data from the ADOPT trial. Following release of this statement, Takeda Pharmaceutical Company, the developer of pioglitazone (sold as Actos in many markets) admitted that it has similar implications for female patients.[10]
The risk of hypoglycemia is low in the absence of other drugs that lower blood glucose.
Pioglitazone can cause fluid retention and peripheral edema. As a result, it may precipitate congestive heart failure (which worsens with fluid overload in those at risk). It may cause anemia. Mild weight gain is common due to increase in subcutaneous adipose tissue. In studies, patients on pioglitazone had an increased proportion of upper respiratory tract infection, sinusitis, headache, myalgia and tooth problems.
Chronic administration of the drug has led to occasional instances of cholestatic hepatitis, reversible upon drug discontinuation.[11]
On July 30, 2007 an Advisory Committee of the Food and Drug Administration concluded that the use of rosiglitazone for the treatment of type 2 diabetes was associated with a greater risk of "myocardial ischemic events" when compared to placebo, but when compared to other diabetes drugs, there was no increased risk. Pioglitazone is currently being reviewed. A meta-analysis released subsequently showed that pioglitazone reduced the risk of ischemic cardiac events rather than increased the risk, but increased CHF.[12]
Bladder cancer
On June 9, 2011 the French Agency for the Safety of Health Products decided to withdraw pioglitazone due to high risk of bladder cancer.[13] This suspension was based on the results of an epidemiological study conducted by the French National Health Insurance. According to the results of the epidemiological study, the French agency found that patients, who were taking Actos for a long time to aid in type 2 diabetes mellitus, significantly increased risk of bladder cancer compared with patients who were taking other diabetes medications.[14] On June 10, 2011 Germany's Federal Institute for Drugs and Medical Devices also advised doctors not to prescribe the medication until further investigation of the cancer risk had been conducted.[15]
On June 15, 2011 the U.S. FDA announced that pioglitazone use for more than one year may be associated with an increased risk of bladder cancer, and two months later the label was updated with an additional warning about this risk.[16]
A 2017 meta-analysis of diabetes found no difference in the rates of bladder cancer attributed to the pioglitazone.[17]
Drug interactions
Combination with sulfonylureas or insulin reciprocally exponentiate risk of hypoglycemia. Therapy with pioglitazone increase the chance of pregnancy in individuals taking oral contraception.
Mechanism of action
Pioglitazone selectively stimulates the nuclear receptor peroxisome proliferator-activated receptor gamma (PPAR-γ) and to a lesser extent PPAR-α.[18][19] It modulates the transcription of the genes involved in the control of glucose and lipid metabolism in the muscle, adipose tissue, and the liver. As a result, pioglitazone reduces insulin resistance in the liver and peripheral tissues, decreases gluconeogenesis in the liver, and reduces quantity of glucose and glycated hemoglobin in the bloodstream.
More recently, pioglitazone and other active TZDs have been shown to bind to the outer mitochondrial membrane protein mitoNEET with affinity comparable to that of pioglitazone for PPARγ.[20][21]
Society and culture
In 2008 it generated the tenth-highest amount of money for a medication in the U.S. in 2008, with sales exceeding $2.4 billion.[22]
Brand names
Pioglitazone is marketed as trademarks Actos in the USA, Canada, the UK and Germany, Glustin in Europe, Glizone and Pioz in India by Zydus Cadila and USV Limited, respectively and Zactos in Mexico by Takeda Pharmaceuticals. On August 17, 2012 the US FDA announced its approval of the first generic version of Actos.[23]
Research
There is tentative research that suggests that pioglitazone may be useful for treating major depression.[24]
Because it is thought to reduce inflammatory activity in neuroglia, it was studied in a small clinical trial involving children with autism, under the autoimmune/inflammatory hypotheses of the causes of autism.[25]
References
- British national formulary : BNF 76 (76 ed.). Pharmaceutical Press. 2018. p. 694. ISBN 9780857113382.
- "Pioglitazone Hydrochloride Monograph for Professionals". Drugs.com. American Society of Health-System Pharmacists. Retrieved 3 March 2019.
- Fischer J, Ganellin CR (2006). Analogue-based Drug Discovery. John Wiley & Sons. p. 450. ISBN 9783527607495.
- "NADAC as of 2019-02-27". Centers for Medicare and Medicaid Services. Retrieved 3 March 2019.
- "The Top 300 of 2020". ClinCalc. Retrieved 11 April 2020.
- "Pioglitazone - Drug Usage Statistics". ClinCalc. Retrieved 11 April 2020.
- Burant C (2012). Medical Management of Type 2 Diabetes. American Diabetes Association. p. 63. ISBN 9781580404570.
- "Actos- pioglitazone tablet". DailyMed. 25 January 2019. Retrieved 13 February 2020.
- Scheen AJ (November 2012). "Outcomes and lessons from the PROactive study". Diabetes Research and Clinical Practice. 98 (2): 175–86. doi:10.1016/j.diabres.2012.09.001. PMID 23020930.
Since 2005, there has been much debate on the relative value of the statistically non-significant 10% reduction in the quite challenging primary composite endpoint (combining cardiovascular disease-driven and procedural events in all vascular beds) versus the statistically significant 16% decrease in the more robust and conventional main secondary endpoint (all-cause mortality, myocardial infarction, and stroke) observed with pioglitazone.
- Takeda (March 2007). "Observation of an Increased Incidence of Fractures in Female Patients Who Received Long-Term Treatment with ACTOSO (pioglitazone HOI) Tablets for Type 2 Diabetes Mellitus" (PDF). Archived from the original (PDF) on 2012-05-16. Retrieved 2012-04-04.
- Baselt R (2008). Disposition of Toxic Drugs and Chemicals in Man (8th ed.). Foster City, CA: Biomedical Publications. pp. 1271–1272.
- Lincoff AM, Wolski K, Nicholls SJ, Nissen SE (September 2007). "Pioglitazone and risk of cardiovascular events in patients with type 2 diabetes mellitus: a meta-analysis of randomized trials". JAMA. 298 (10): 1180–8. doi:10.1001/jama.298.10.1180. PMID 17848652.
- "L'antidiabétique Actos retiré du marché" [Actos antidiabetic withdrawn from the market]. Le Figaro (in French). 9 June 2011.
- Alam II (1 January 2012). "France and Germany Suspended Use of Actos for Bladder Cancer Risk". Medical-Reference.
- Topham J (June 10, 2011). "UPDATE 2-Germany joins France in suspending top Takeda drug". Reuters.
- "FDA Drug Safety Communication: Updated drug labels for pioglitazone-containing medicines". United States Food and Drug Administration. 4 August 2011.
- Filipova E, Uzunova K, Kalinov K, Vekov T (August 2017). "Pioglitazone and the Risk of Bladder Cancer: A Meta-Analysis". Diabetes Therapy. 8 (4): 705–726. doi:10.1007/s13300-017-0273-4. PMC 5544610. PMID 28623552.
- Gillies PS, Dunn CJ (August 2000). "Pioglitazone". Drugs. 60 (2): 333–43, discussion 344–5. doi:10.2165/00003495-200060020-00009. PMID 10983737.
- Smith U (September 2001). "Pioglitazone: mechanism of action". International Journal of Clinical Practice. Supplement (121): 13–8. PMID 11594239.
- Colca JR, McDonald WG, Waldon DJ, Leone JW, Lull JM, Bannow CA, Lund ET, Mathews WR (February 2004). "Identification of a novel mitochondrial protein ("mitoNEET") cross-linked specifically by a thiazolidinedione photoprobe". American Journal of Physiology. Endocrinology and Metabolism. 286 (2): E252–60. doi:10.1152/ajpendo.00424.2003. PMID 14570702.
- Paddock ML, Wiley SE, Axelrod HL, Cohen AE, Roy M, Abresch EC, Capraro D, Murphy AN, Nechushtai R, Dixon JE, Jennings PA (September 2007). "MitoNEET is a uniquely folded 2Fe 2S outer mitochondrial membrane protein stabilized by pioglitazone". Proceedings of the National Academy of Sciences of the United States of America. 104 (36): 14342–7. Bibcode:2007PNAS..10414342P. doi:10.1073/pnas.0707189104. PMC 1963346. PMID 17766440.
- "Details for Actos". Drug Patent Watch.
- "FDA approves first generic Actos to treat type 2 diabetes". United States Food and Drug Administration. 17 August 2012. Archived from the original on 4 January 2016.
- Colle R, de Larminat D, Rotenberg S, Hozer F, Hardy P, Verstuyft C, Fève B, Corruble E (2017). "Pioglitazone could induce remission in major depression: a meta-analysis". Neuropsychiatric Disease and Treatment. 13: 9–16. doi:10.2147/NDT.S121149. PMC 5182046. PMID 28031713.
- Doyle CA, McDougle CJ (August 2012). "Pharmacotherapy to control behavioral symptoms in children with autism". Expert Opinion on Pharmacotherapy. 13 (11): 1615–29. doi:10.1517/14656566.2012.674110. PMID 22550944.
- Pietrzak A, Michalak-Stoma A, Chodorowska G, Szepietowski JC (2010). "Lipid disturbances in psoriasis: an update". Mediators of Inflammation. 2010: 535612. doi:10.1155/2010/535612. PMC 2914266. PMID 20706605.