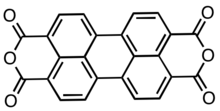
Perylenetetracarboxylic dianhydride
Perylenetetracarboxylic dianhydride (PTCDA) is an organic dye molecule and an organic semiconductor. It is used as a precursor to a class of molecules known as Rylene dyes, which are useful as pigments and dyes. It is a dark red solid with low solubility in aromatic solvents. The compound has attracted much interest as an organic semiconductor.
 | |
| Names | |
|---|---|
| Other names
Perylene-3,4,9,10-tetracarboxylic dianhydride, Pigment Red 224 | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.004.461 |
| EC Number |
|
PubChem CID |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C24H8O6 | |
| Molar mass | 392.32 |
| Density | 1.7 g/cm3 |
| Melting point | ~350 °C[1] |
| Structure | |
| Monoclinic, P21/c | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Structure
PTCDA consists of a perylene core to which two anhydride groups have been attached, one at either side. It occurs in two crystalline forms, α and β.[2] Both have the P21/c monoclinic symmetry and a density of ca. 1.7 g/cm3, which is relatively high for organic compounds. Their lattice parameters are:
| Form | a | b | c | γ |
|---|---|---|---|---|
| α | 0.374 nm | 1.196 nm | 1.734 nm | 98.8° |
| β | 0.378 nm | 1.930 nm | 1.077 nm | 83.6° |
Self-assembly and films

Atomic force microscopy image of a single PTCDA molecule on Si at room temperature.[3]
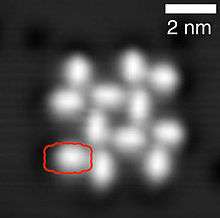
Self-assembly of PTCDA molecules on NaCl, scanning tunneling microscopy image.[4]
Use
The main industrial use of PTCDA is as a precursor to Rylene dyes.[5][6]
References
| Wikimedia Commons has media related to PTCDA. |
- PTCDA.
- Möbus, M. & Karl, N. (1992). "Structure of perylene-tetracarboxylic-dianhydride thin films on alkali halide crystal substrates". Journal of Crystal Growth. 116 (3–4): 495–504. doi:10.1016/0022-0248(92)90658-6.
- Iwata, Kota; Yamazaki, Shiro; Mutombo, Pingo; Hapala, Prokop; Ondráček, Martin; Jelínek, Pavel; Sugimoto, Yoshiaki (2015). "Chemical structure imaging of a single molecule by atomic force microscopy at room temperature". Nature Communications. 6: 7766. doi:10.1038/ncomms8766. PMC 4518281. PMID 26178193.
- Cochrane, K. A.; Schiffrin, A.; Roussy, T. S.; Capsoni, M.; Burke, S. A. (2015). "Pronounced polarization-induced energy level shifts at boundaries of organic semiconductor nanostructures". Nature Communications. 6: 8312. doi:10.1038/ncomms9312. PMC 4600718. PMID 26440933.
- Hunger, K. and Herbst, W. (2012) "Pigments, Organic" in Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH, Weinheim. doi:10.1002/14356007.a20_371
- Greene, M. (2009) "Perylene Pigments", pp. 261–274 in High Performance Pigments, Wiley-VCH, Weinheim.doi:10.1002/9783527626915.ch16
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.
