Ciclesonide
Ciclesonide is a glucocorticoid used to treat asthma and allergic rhinitis. It is marketed under the brand names Alvesco for asthma and Omnaris, Omniair, Zetonna, and Alvesco[1] for hay fever in the US and Canada.
 | |
 | |
| Clinical data | |
|---|---|
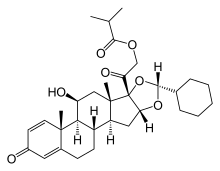
| Other names | (11β, 16α)-16, 17-[[(R)-cyclohexylmethylene]bis(oxy)]-11-hydroxy-21- (2-methyl-1-oxopropoxy)- pregna-1, 4-diene-3, 20-dione |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a607008 |
| Pregnancy category | |
| Routes of administration | Nasal inhalation |
| ATC code | |
| Legal status | |
| Legal status | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.210.908 |
| Chemical and physical data | |
| Formula | C32H44O7 |
| Molar mass | 540.697 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Side effects of the medication include headache, nosebleeds, and inflammation of the nose and throat linings.[2]
It was patented in 1990 and approved for medical use in 2005.[3] The drug was approved for adults and children 12 and over by the US Food and Drug Administration in October 2006.[4]
See also
- Beclometasone dipropionate
References
- "Covis Pharma – Products".
- Mutch E, Nave R, McCracken N, Zech K, Williams FM (May 2007). "The role of esterases in the metabolism of ciclesonide to desisobutyryl-ciclesonide in human tissue". Biochemical Pharmacology. 73 (10): 1657–64. doi:10.1016/j.bcp.2007.01.031. PMID 17331475.
- Fischer J, Ganellin CR (2006). Analogue-based Drug Discovery. John Wiley & Sons. p. 488. ISBN 9783527607495.
- "FDA News Release. FDA Approves New Treatment for Allergies". Food and Drug Administration. 2006-10-23. Retrieved 2009-07-30.
Further reading
- Rossi S, ed. (2006). Australian Medicines Handbook. Adelaide: Australian Medicines Handbook. ISBN 0-9757919-2-3.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.