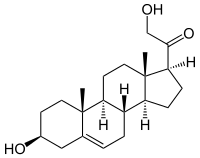
21-Hydroxypregnenolone
21-Hydroxypregnenolone, also known as prebediolone, as well as 3β,21-dihydroxypregn-5-en-20-one, is a naturally occurring and endogenous pregnane steroid, and an intermediate in the biosynthesis of 11-deoxycorticosterone (21-hydroxyprogesterone), corticosterone (11β,21-dihydroxyprogesterone), and other corticosteroids.[1] It is formed from pregnenolone in the adrenal glands.[1]
 | |
 | |
| Names | |
|---|---|
| IUPAC name
2-Hydroxy-1-[(3S,8S,9S,10R,13S,14S,17S)-3-hydroxy-10,13-dimethyl-2,3,4,7,8,9,11,12,14,15,16,17-dodecahydro-1H-cyclopenta[a]phenanthren-17-yl]ethanone | |
| Other names
Prebediolone; 3β,21-Dihydroxypregn-5-en-20-one; Pregn-5-en-3β,21-diol-20-one | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
PubChem CID |
|
| UNII | |
| |
| |
| Properties | |
| C21H32O3 | |
| Molar mass | 332.484 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
The 21-acetate ester of 21-hydroxypregnenolone, prebediolone acetate, is described as a glucocorticoid and has been used in the treatment of rheumatoid arthritis.[2][3][4]
References
- http://www.hmdb.ca/metabolites/HMDB04026
- J. Elks (14 November 2014). The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. pp. 665–. ISBN 978-1-4757-2085-3.
- BRUGSCH HG, MANNING RA (1951). "A comparative study of pregnenolone, 21-acetoxypregnenolone and ACTH". N. Engl. J. Med. 244 (17): 628–32. doi:10.1056/NEJM195104262441703. PMID 14815736.
- LEFKOVITS AM (1953). "Artisone therapy in rheumatoid arthritis". Rheumatism. 9 (4): 70–6. PMID 13101380.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.