Manganese(II) iodide
Manganese(II) iodide is the chemical compound composed of manganese and iodine with the formula MnI2.
 | |
| Names | |
|---|---|
| IUPAC name
Manganese(II) iodide | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.029.274 |
| EC Number |
|
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| MnI2 | |
| Molar mass | 308.747 g/mol |
| Appearance | pink crystalline (looks like MnBr2) |
| Density | 5.01 g/cm3 |
| Melting point | 701 °C (1,294 °F; 974 K) (anhydrous) 80 °C (tetrahydrate) |
| Boiling point | 1,033 °C (1,891 °F; 1,306 K) |
| soluble | |
| +14,400·10−6 cm3/mol | |
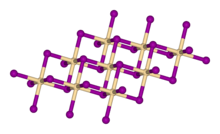
| Structure | |
| Rhombohedral, hP3, SpaceGroup = P-3m1, No. 164 | |
| octahedral | |
| Hazards | |
EU classification (DSD) (outdated) |
Harmful (Xn) |
| R-phrases (outdated) | R20/21/22 |
| S-phrases (outdated) | S36[1] |
| NFPA 704 (fire diamond) | |
| Flash point | non-flammable |
| Related compounds | |
Other anions |
Manganese(II) fluoride Manganese(II) chloride Manganese(II) bromide |
Other cations |
Iron(II) iodide Cobalt(II) iodide |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
It can be used as a pink pigment or as a source of the manganese ion or iodide ion. It is often used in the lighting industry.[2]
References
- "223646 Manganese(II) iodide 98%". Sigma-Aldrich. Retrieved 2011-08-05.
- Cepanec, Ivica (2004). Synthesis of Biaryls. Elseveir. p. 104. ISBN 0-08-044412-1. Retrieved 2008-06-18.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.
