Mandelic acid
Mandelic acid is an aromatic alpha hydroxy acid with the molecular formula C6H5CH(OH)CO2H. It is a white crystalline solid that is soluble in water and polar organic solvents. It is a useful precursor to various drugs. The molecule is chiral. The racemic mixture is known as paramandelic acid.
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
Hydroxy(phenyl)acetic acid | |||
| Other names
2-Hydroxy-2-phenylacetic acid Mandelic acid Phenylglycolic acid α-Hydroxyphenylacetic acid | |||
| Identifiers | |||
| |||
3D model (JSmol) |
|||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.001.825 | ||
| EC Number |
| ||
PubChem CID |
|||
| RTECS number |
| ||
| UNII | |||
CompTox Dashboard (EPA) |
|||
| |||
| |||
| Properties | |||
| C8H8O3 | |||
| Molar mass | 152.149 g·mol−1 | ||
| Appearance | White crystalline powder | ||
| Density | 1.30 g/cm3 | ||
| Melting point | 119 °C (246 °F; 392 K) optically pure: 132 to 135 °C (270 to 275 °F; 405 to 408 K) | ||
| Boiling point | 321.8 °C (611.2 °F; 595.0 K) | ||
| 15.87 g/100 mL | |||
| Solubility | soluble in diethyl ether, ethanol, isopropanol | ||
| Acidity (pKa) | 3.41[2] | ||
Refractive index (nD) |
1.5204 | ||
| Thermochemistry | |||
Std enthalpy of formation (ΔfH⦵298) |
0.1761 kJ/g | ||
| Pharmacology | |||
| B05CA06 (WHO) J01XX06 (WHO) | |||
| Hazards | |||
| Flash point | 162.6 °C (324.7 °F; 435.8 K) | ||
| Related compounds | |||
Related compounds |
mandelonitrile, phenylacetic acid, vanillylmandelic acid | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
Isolation, synthesis, occurrence
Mandelic acid was discovered in 1831 by the German pharmacist Ferdinand Ludwig Winckler (1801–1868) while heating amygdalin, an extract of bitter almonds, with diluted hydrochloric acid.[3] The name is derived from the German "Mandel" for "almond". Derivatives of mandelic acid are formed as a result of metabolism of adrenaline and noradrenaline by monoamine oxidase and catechol-O-methyl transferase.
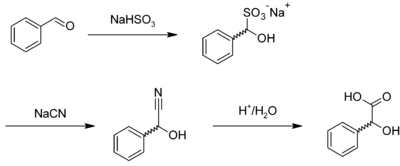
Mandelic acid is usually prepared by the acid-catalysed hydrolysis of mandelonitrile,[4] which is the cyanohydrin of benzaldehyde. Mandelonitrile can also be prepared by reacting benzaldehyde with sodium bisulfite to give the corresponding adduct, forming mandelonitrile with sodium cyanide, which is hydrolyzed:[5]
Alternatively, it can be prepared by base hydrolysis of phenylchloroacetic acid and dibromacetophenone.[6] It also arises by heating phenylglyoxal with alkalis.[7][8]
The biotechnological production of 4-hydroxy-mandelic acid and mandelic acid on the basis of glucose was demonstrated with a genetically modified yeast Saccharomyces cerevisiae, in which the hydroxymandelate synthase naturally occurring in the bacterium Amycolatopsis was incorporated into a wild-type strain of yeast, partially altered by the exchange of a gene sequence and expressed.[9]
It also arises from the biodegradation of styrene, as detected in urine.[10]
Uses
Mandelic acid has a long history of use in the medical community as an antibacterial, particularly in the treatment of urinary tract infections.[11] It has also been used as an oral antibiotic, and as a component of chemical face peels analogous to other alpha hydroxy acids.[12]
The drugs cyclandelate and homatropine are esters of mandelic acid.
References
- Merck Index, 11th Edition, 5599.
- Bjerrum, J., et al. Stability Constants, Chemical Society, London, 1958.
- See:
- Winckler, F. L. (1831) "Ueber die Zersetzung des Calomels durch Bittermandelwasser, und einige Beiträge zur genaueren Kenntniss der chemischen Zusammensetzung des Bittermandelwassers" (On the decomposition of calomel [i.e., mercury(I) chloride] by bitter almond water, and some contributions to a more precise knowledge of the chemical composition of bitter almond water), Repertorium für die Pharmacie, 37 : 388–418 ; mandelic acid is named on p. 415.
- Winckler, F. L. (1831) "Ueber die chemische Zusammensetzung des Bittermandelwassers; als Fortsetzung der im 37sten Band S. 388 u.s.w. des Repertoriums enthaltenen Mittheilungen" [On the chemical composition of bitter almond water; as a continuation of the report contained in the 37th volume, pp. 388 ff. of the Repertorium], Repertorium für die Pharmacie, 38 : 169–196. On p. 193, Winckler describes the preparation of mandelic acid from bitter almond water and hydrochloric acid (Salzsäure).
- (Editor) (1832) "Ueber einige Bestandtheile der Bittermandeln" (On some components of bitter almonds), Annalen der Chemie und Pharmacie, 4 : 242–247.
- Winckler, F. L. (1836) "Ueber die Mandelsäure und einige Salze derselben" (On mandelic acid and some salts of the same), Annalen der Chemie und Pharmacie, 18 (3) : 310–319.
- Hermann Schelenz, Geschichte der Pharmazie [The History of Pharmacy] (Berlin, German: Julius Springer, 1904), p. 675.
- Edwin Ritzer and Rudolf Sundermann "Hydroxycarboxylic Acids, Aromatic" in Ullmann's Encyclopedia of Industrial Chemistry 2002, Wiley-VCH, Weinheim. doi: 10.1002/14356007.a13_519
- Corson, B. B.; Dodge, R. A.; Harris, S. A.; Yeaw, J. S. (1926). "Mandelic Acid". Org. Synth. 6: 58. doi:10.15227/orgsyn.006.0058.CS1 maint: multiple names: authors list (link)
- J. G. Aston, J. D. Newkirk, D. M. Jenkins, and Julian Dorsky (1952). "Mandelic Acid". Organic Syntheses.CS1 maint: multiple names: authors list (link); Collective Volume, 3, p. 538
- Pechmann, H. von (1887). "Zur Spaltung der Isonitrosoverbindungen". Berichte der Deutschen Chemischen Gesellschaft. 20 (2): 2904–2906. doi:10.1002/cber.188702002156.
- Pechmann, H. von; Muller, Hermann (1889). "Ueber α-Ketoaldehyde". Berichte der Deutschen Chemischen Gesellschaft. 22 (2): 2556–2561. doi:10.1002/cber.188902202145.
- Mara Reifenrath, Eckhard Boles: Engineering of hydroxymandelate synthases and the aromatic amino acid pathway enables de novo biosynthesis of mandelic and 4-hydroxymandelic acid with Saccharomyces cerevisiae. Metabolic Engineering 45, Januar 2018; S. 246-254. doi:10.1016/j.ymben.2018.01.001.
- Engström K, Härkönen H, Kalliokoski P, Rantanen J. "Urinary mandelic acid concentration after occupational exposure to styrene and its use as a biological exposure test" Scand. J. Work Environ. Health. 1976, volume 2, pp. 21-6.
- Putten, P. L. (1979). "Mandelic acid and urinary tract infections". Antonie van Leeuwenhoek. 45 (4): 622–623. doi:10.1007/BF00403669.
- Taylor, MB. (1999). "Summary of mandelic acid for the improvement of skin conditions". Cosmetic Dermatology. 21: 26–28.

-Mandelic_acid_molecule_ball.png)