Lumateperone
Lumateperone (INN; brand name Caplyta kəp-LY-tə, developmental codes ITI-007 and ITI-722) is a butyrophenone atypical antipsychotic developed by Intra-Cellular Therapies, licensed from Bristol-Myers Squibb, for the treatment of schizophrenia,[1] and currently in development for bipolar depression and other neurological indications.[2]
 | |
| Clinical data | |
|---|---|
| Pronunciation | /luːməˈtɛpərɑːn/ loo-mə-TE-pə-ron |
| Trade names | Caplyta |
| Other names | ITI-007; ITI-722 |
| Routes of administration | By mouth |
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| Chemical and physical data | |
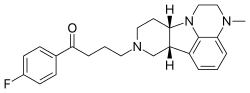
| Formula | C24H28FN3O |
| Molar mass | 393.506 g·mol−1 |
| 3D model (JSmol) | |
| |
Medical uses
Schizophrenia
On December 20, 2019, the United States Food and Drug Administration approved lumateperone for use in adult schizophrenic patients.[3] The drug is expected to be ready for use in Spring of 2020.
Clinical Studies
Bipolar depression
Two phase III lumateperone monotherapy studies were conducted and completed for the treatment of bipolar depression, those being trial Study 401 and Study 404.[4] A third trial, Study 402, aims to test lumateperone in addition to lithium or valproate,[5][6] the data pertaining this trial is due out in 2020.[7][8]
Study 401 was conducted solely in the United States while Study 404 was a global study and included patients from the US.[9][10] Of the entire Study 404 population (381 patients), two-thirds were from Russia and Colombia. At the completion of the two monotherapy phase III trials only Study 404 met its primary endpoint and one of its secondary endpoints.[11][12] In Study 404, patients received 42 mg lumateperone once daily or placebo for six weeks. Study 404 patients saw an improvement of depressive symptoms compared to placebo as documented by a change in MADRS total score of 4.6.[13]
Pharmacology
Mechanism of action
| Receptor | Ki (nM) | |
|---|---|---|
| 5-HT2A | 0 |
.54 |
| Dopamine receptor D1 | 52 | |
| Dopamine receptor D2 | 32 | |
Lumateperone affects multiple systems (serotonergic, dopaminergic and glutamatergic) in the central nervous system. It acts as a antagonist of 5-HT2A receptor and modulates D1 and D2 dopamine receptors where it is a partial agonist at presynaptic and partial antagonist at postsynaptic receptors. Through the mTOR pathway, lumateperone augments both NMDA and AMPA activity. Because of its complex pharmacology, it is not clear which of these activities are primarily responsible for its antidepressive and antipsychotic activities.[15]
Pharmacokinetics
After taking the medication by mouth, lumateperone reaches maximum plasma concentrations within 3-4 hours and has an elimination half-life of 13 hours. The main metabolite is the reduction of the carbonyl to hydroxyl group by a ketone reductase. The N-methyl group is also dealkylated by CYP3A4.[16]
Society and culture
References
- Celanire S, Poli S, eds. (13 October 2014). Small Molecule Therapeutics for Schizophrenia. Springer. pp. 31–. ISBN 978-3-319-11502-3.
- "Another blow for Intra-Cellular". Evaluate.com. 24 July 2019. Retrieved 6 November 2019.
- "FDA Approves Caplyta (lumateperone) for the Treatment of Schizophrenia in Adults". drugs.com. 23 December 2019.
- Intra-Cellular Therapies Inc. (8 July 2019). "Intra-Cellular Therapies Announces Positive Top-line Results from a Phase 3 Trial of Lumateperone in Patients with Bipolar Depression". GlobeNewswire News Room. Retrieved 6 November 2019.
- Intra-Cellular Therapies Inc. (8 July 2019). "Intra-Cellular Therapies Announces Positive Top-line Results from a Phase 3 Trial of Lumateperone in Patients with Bipolar Depression". GlobeNewswire News Room. Retrieved 6 November 2019.
- "Why Intra-Cellular Therapies Is Tanking Today". finance.yahoo.com. Retrieved 6 November 2019.
- "One out of two is not enough for Intra-Cellular". Evaluate.com. 8 July 2019. Retrieved 6 November 2019.
- "Why Intra-Cellular Therapies Is Tanking Today". finance.yahoo.com. Retrieved 6 November 2019.
- "Intra-Cellular Therapies Announces Top Line Results for Two Bipolar Studies". Trial Site News. 13 July 2019. Retrieved 6 November 2019.
- Intra-Cellular Therapies Inc. (8 July 2019). "Intra-Cellular Therapies Announces Positive Top-line Results from a Phase 3 Trial of Lumateperone in Patients with Bipolar Depression". GlobeNewswire News Room. Retrieved 6 November 2019.
- "One out of two is not enough for Intra-Cellular". Evaluate.com. 8 July 2019. Retrieved 6 November 2019.
- DeArment A (8 July 2019). "Intra-Cellular Therapies hits one, misses another in Phase III bipolar disorder program". MedCity News. Retrieved 6 November 2019.
- "Phase 3 data supports lumateperone for bipolar depression". www.healio.com. 8 July 2019. Retrieved 6 November 2019.
- Davis RE, Correll CU (June 2016). "ITI-007 in the treatment of schizophrenia: from novel pharmacology to clinical outcomes". Expert Review of Neurotherapeutics. 16 (6): 601–14. doi:10.1080/14737175.2016.1174577. PMID 27042868.
- Kumar B, Kuhad A, Kuhad A (December 2018). "Lumateperone: a new treatment approach for neuropsychiatric disorders". Drugs of Today. Barcelona, Spain. 54 (12): 713–719. doi:10.1358/dot.2018.54.12.2899443. PMID 30596390.
- Vyas P, Hwang BJ, Brašić JR (November 2019). "An evaluation of lumateperone tosylate for the treatment of schizophrenia". Expert Opinion on Pharmacotherapy: 1–7. doi:10.1080/14656566.2019.1695778. PMID 31790322.
- House DW, ed. (8 July 2019). "Intra-Cellular down 9% premarket on uneven results from lumateperone studies". Seeking Alpha. Retrieved 6 November 2019.
- "Why Intra-Cellular Therapies Is Tanking Today". finance.yahoo.com. Retrieved 6 November 2019.
- "Lumateperone schizophrenia drug seems to hit snag". www.mdedge.com. Retrieved 6 November 2019.
- "Lumateperone for schizophrenia shows safety, tolerability in long-term study". www.mdedge.com. Retrieved 6 November 2019.
External links
- "Lumateperone". Drug Information Portal. U.S. National Library of Medicine.
- "Drug Trials Snapshots: Caplyta". U.S. Food and Drug Administration (FDA). 20 December 2019. Retrieved 23 January 2020.</ref>