Krische allylation
The Krische allylation involves the iridium-catalyzed enantioselective addition of an allyl group to an aldehyde or an alcohol, resulting in the formation of a secondary alcohol and a new carbon-carbon bond.[1][2] This method of asymmetric carbonyl allylation employs catalytic chiral iridium catalysts that can be generated in situ, thereby avoiding the necessity for preformed stoichiometric allyl metal reagents and stoichiometric chiral reagents, and significantly reducing waste production. The mechanism of the Krische allylation involves transfer hydrogenation, precluding the use of redox manipulations (alcohol to aldehyde oxidation) that are generally needed for such transformations.[1][3]
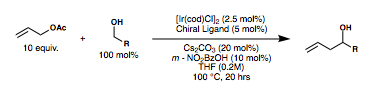
Background
Enantioselective carbonyl allylations are significant for their application to the synthesis of polyketide natural products. In 1978, Hoffman described the first chiral allyl metal reagent, an allylborane derived from camphor.[4][5] Following his discovery, improved methods for the asymmetric carbonyl allylation emerged from Kumada, Roush, Brown, Leighton, and others.[6][7][8][9][10][11] Unfortunately, these carbon-carbon bond forming reactions have historically resulted in the formation of undesired and stoichiometric metal byproducts due to the necessity of preformed allyl metal reagents as allyl donors.
In 1991, Yamamoto paved the way for the first catalytic enantioselective methodologies with his Lewis acid-catalyzed carbonyl allylation. Such catalytic methods would circumvent the formation of the stoichiometric byproducts.[12] His work was followed by Umani-Ronchi[13] and Keck,[14] who also contributed enantioselective catalytic carbonyl allylation methodologies. While these contributions to the field were impactful, they did not circumvent the need for pre-formed allyl metal reagents. Catalytic variants of the Nozaki-Hiyama-Kishi reaction present another related method for carbonyl allylation, but they still rely on the use of stoichiometric metallic reductants to turn over the catalytic cycle.[15] In addition to the previously stated drawbacks of these allylation methods, many of them also require stoichiometric chiral starting materials (i.e. pinene, tartrates, etc.), anhydrous reaction conditions, and/or pre-formation of reagents that are not trivial to make or to work with. Polyketide synthesis based on first-generation technologies described above often end up being too long for large scale application due to the need for added redox manipulations (i.e. alcohol to carbonyl oxidations) and protecting group requirements.
Reaction features
The Krische allylation sets out to bypass some of these drawbacks through transfer hydrogenative carbon-carbon bond formation.[3][1] In a series of papers published in the early 2000s, Krische and coworkers developed a family of catalytic carbonyl allylation methods in which allenes, dienes, and allyl acetate are able to function as allyl donors and precursors to transient allyl metal nucleophiles.[16][17][18][19][20][21] Notably, this work allows for enantioselective carbonyl allylation without pre-formed organometallic reagents or metallic reductants. This circumvents additional preparative steps and the formation of undesired byproducts. An important feature of this reaction is that it can be done from the alcohol or aldehyde oxidation state without the usual need for redox manipulations. In the case of the aldehyde, isopropanol is added as a terminal reductant to turn over the metal catalyst. Remarkably, due to a kinetic preference for primary alcohols, protecting group manipulations are not necessary when performing this reaction on a substrate that contains both primary and secondary alcohols.[22]
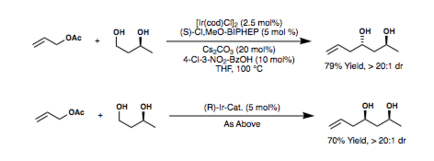
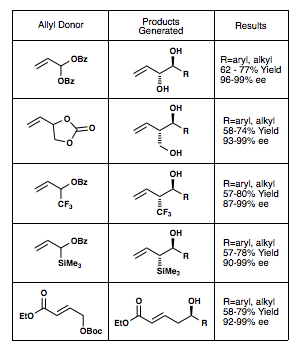
The figure below shows the different allyl donors[23][24][25][26][27] that have been successful in the Krische allylation reaction, along with their respective yields and enantiomeric excess.
It is important to note that, although the Krische allylation addresses several key inconveniences of earlier allylation methods, the conditions of this reaction are often considered to be harsh. The use of high temperatures and the need for base in the reaction maybe lower the functional group tolerance in some cases.
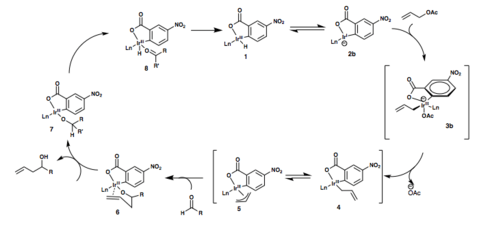
Mechanism
The following two mechanisms have been postulated for Krische’s iridium-catalyzed transfer hydrogenative carbonyl allylation. In both mechanisms, in situ formation of the active catalyst occurs first through the complexation the chelating phosphine ligand and m-NO2BzOH with [Ir(cod)Cl]2 to give Iridium carboxylate complex 2a. This complex is proposed to be in equilibrium with the ortho-cyclometallated complex 1.[1]
Oxidative addition of allyl acetate to the iridium is immediately followed by ortho-metalation and formation of the six-membered transition state, 3a. Loss of acetic acid then gives the sigma-allyl C,O-benzoate complex 4, which quickly equilibrates to the pi-allyl haptomer 5.
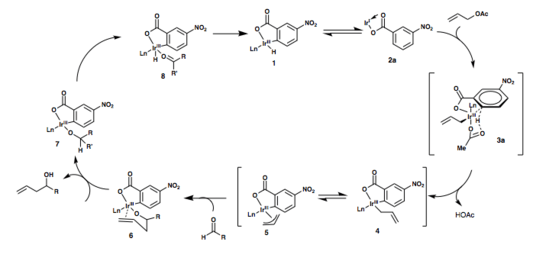
Coordination of the aldehyde to the iridium results in the formation of a closed chair-like transition state with the allyl moiety, followed by generation of the homoallyl iridium alkoxide 6. This species is presumed to be stable due to coordination of the double bond with the metal, disabling beta-hydride elimination. Exchange of the homoallyl alcohol with another molecule of the reactant alcohol or with another alcohol (isopropanol, or an alcohol that can act as a terminal reductant and turn over the catalytic cycle) opens a coordination site on the iridium (7) and allows for beta-hydride elimination, giving 8 and turning over the catalytic cycle. Finally, dissociation of the aldehyde gives the starting catalyst complex 1.[1]
Another plausible mechanism is shown in the second figure, shown below. Proton loss from complex 1, followed by oxidative addition of allyl acetate to the iridium gives complex 3b. Loss of the acetate anion results in formation of complexes 4 and 5, after which the catalytic cycle is identical to the one described previously.[1]
Catalysts
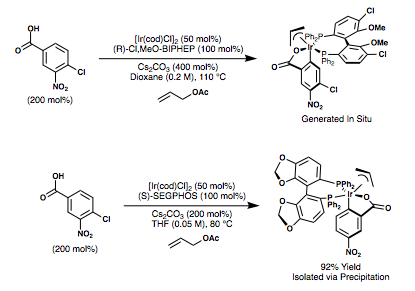
Iridium and ruthenium complexes are used in these metal-catalyzed transfer hydrogenative asymmetric alcohol C-C couplings. Krische and coworkers have developed cyclometalated π-allyliridium(III) ortho-C,O-benzoate complexes derived from [Ir(cod)Cl]2, allyl acetate, various 4-substituted-3-nitro-benzoic acids, and axially chiral bis(phosphine) ligands for these transformations. These catalyst complexes are often generated in situ, but they can also be pre-generated and isolated as they are robust and air-stable. Shown below are syntheses of two catalysts used for these transformations.[28]
Applications in Synthesis
Krische and others have applied this iridium-catalyzed, transfer-hydrogenative carbonyl allylation method to the synthesis of polyketide natural products. Some examples are shown below. In every case, the target compound was prepared in significantly fewer steps than was previously achieved.
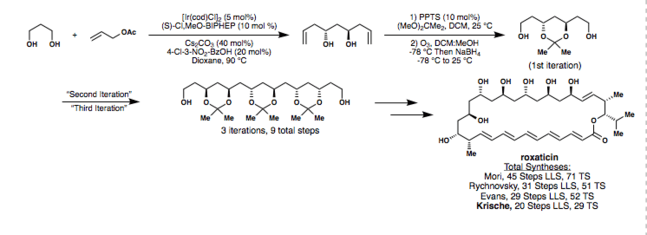
In his synthesis of roxaticin, Krische repeated a sequence including his bisallylation, followed by acetonide formation, and then ozonolysis in three consecutive iterations.[29] The synthesis was completed in a significantly lower number of steps than had ever been achieved before,[30][31][32][33] made possible in part by Krische's use of his allylation methods. This is shown in the scheme below.
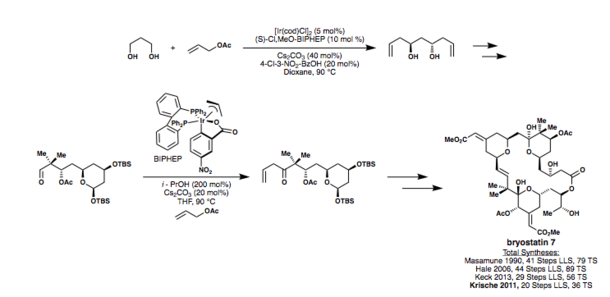
The synthetic utility of these methods is also shown in Krische's synthesis[34] of bryostatin 7, a more efficient synthesis than those of Hale[35] (2008), Keck (2013), Masamune[36] (1990), and others.
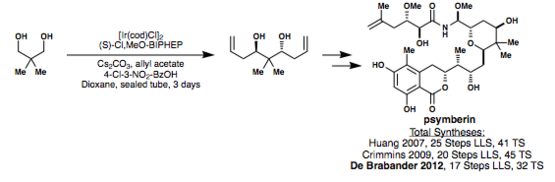
In 2012, De Brabander applied the Krische bisallylation to his synthesis of psymberin in 17 LLS and 32 total steps, as shown below.[37] Through the use of the Krische allylation, this synthesis was accomplished via a much shorter route than previous syntheses of the molecule.
In 2016, Tao employed the Krische allylation to his synthesis of callyspongiolide using the chiral SEGPHOS catalyst complex.[38]
References
- Kim, In Su; Ngai, Ming-Yu; Krische, Michael J. (2008-11-05). "Enantioselective Iridium-Catalyzed Carbonyl Allylation from the Alcohol or Aldehyde Oxidation Level via Transfer Hydrogenative Coupling of Allyl Acetate: Departure from Chirally Modified Allyl Metal Reagents in Carbonyl Addition". Journal of the American Chemical Society. 130 (44): 14891–14899. doi:10.1021/ja805722e. PMC 2890235. PMID 18841896.
- Strategies and Tactics in Organic Synthesis, Volume 10 Michael Harmata Ed.
- Feng, Jiajie; Kasun, Zachary A.; Krische, Michael J. (2016-05-04). "Enantioselective Alcohol C–H Functionalization for Polyketide Construction: Unlocking Redox-Economy and Site-Selectivity for Ideal Chemical Synthesis". Journal of the American Chemical Society. 138 (17): 5467–5478. doi:10.1021/jacs.6b02019. PMC 4871165. PMID 27113543.
- Herold, Thomas; Hoffmann, Reinhard W. (1978-10-01). "Enantioselective Synthesis of Homoallyl Alcohols via Chiral Allylboronic Esters". Angewandte Chemie International Edition in English. 17 (10): 768–769. doi:10.1002/anie.197807682.
- Hoffmann, Reinhard W.; Herold, Thomas (1981-01-01). "Stereoselektive Synthese von Alkoholen, VII1) Optisch aktive Homoallylalkohole durch Addition chiraler Boronsäureester an Aldehyde". Chemische Berichte. 114 (1): 375–383. doi:10.1002/cber.19811140139.
- Hayashi, Tamio; Konishi, Mitsuo; Kumada, Makoto (1982-09-01). "Optically active allylsilanes. 2. High stereoselectivity in asymmetric reaction with aldehydes producing homoallylic alcohols". Journal of the American Chemical Society. 104 (18): 4963–4965. doi:10.1021/ja00382a046.
- Brown, Herbert C.; Jadhav, Prabhakar K. (1983-04-01). "Asymmetric carbon-carbon bond formation via .beta.-allyldiisopinocampheylborane. Simple synthesis of secondary homoallylic alcohols with excellent enantiomeric purities". Journal of the American Chemical Society. 105 (7): 2092–2093. doi:10.1021/ja00345a085.
- Roush, William R.; Walts, Alan E.; Hoong, Lee K. (1985-12-01). "Diastereo- and enantioselective aldehyde addition reactions of 2-allyl-1,3,2-dioxaborolane-4,5-dicarboxylic esters, a useful class of tartrate ester modified allylboronates". Journal of the American Chemical Society. 107 (26): 8186–8190. doi:10.1021/ja00312a062.
- Kinnaird, James W. A.; Ng, Pui Yee; Kubota, Katsumi; Wang, Xiaolun; Leighton, James L. (2002-07-01). "Strained Silacycles in Organic Synthesis: A New Reagent for the Enantioselective Allylation of Aldehydes". Journal of the American Chemical Society. 124 (27): 7920–7921. doi:10.1021/ja0264908.
- Short, Robert P.; Masamune, Satoru (1989-03-01). "Asymmetric allylboration with B-allyl-2-(trimethylsilyl)borolane". Journal of the American Chemical Society. 111 (5): 1892–1894. doi:10.1021/ja00187a061.
- Corey, E. J.; Yu, Chan Mo; Kim, Sung Soo (1989-07-01). "A practical and efficient method for enantioselective allylation of aldehydes". Journal of the American Chemical Society. 111 (14): 5495–5496. doi:10.1021/ja00196a082.
- Furuta, Kyoji; Mouri, Makoto; Yamamoto, Hisashi (1991-01-01). "Chiral (Acyloxy)borane Catalyzed Asymmetric Allylation of Aldehydes". Synlett. 1991 (8): 561–562. doi:10.1055/s-1991-20797.
- Costa, Anna Luisa; Piazza, Maria Giulia; Tagliavini, Emilio; Trombini, Claudio; Umani-Ronchi, Achille (1993-07-01). "Catalytic asymmetric synthesis of homoallylic alcohols". Journal of the American Chemical Society. 115 (15): 7001–7002. doi:10.1021/ja00068a079.
- Keck, Gary E.; Tarbet, Kenneth H.; Geraci, Leo S. (1993-09-01). "Catalytic asymmetric allylation of aldehydes". Journal of the American Chemical Society. 115 (18): 8467–8468. doi:10.1021/ja00071a074.
- Hargaden, Gráinne C.; Guiry, Patrick J. (2007-11-05). "The Development of the Asymmetric Nozaki–Hiyama–Kishi Reaction". Advanced Synthesis & Catalysis. 349 (16): 2407–2424. doi:10.1002/adsc.200700324.
- Skucas, Eduardas; Bower, John F.; Krische, Michael J. (2007-10-01). "Carbonyl Allylation in the Absence of Preformed Allyl Metal Reagents: Reverse Prenylation via Iridium-Catalyzed Hydrogenative Coupling of Dimethylallene". Journal of the American Chemical Society. 129 (42): 12678–12679. doi:10.1021/ja075971u.
- Bower, John F.; Skucas, Eduardas; Patman, Ryan L.; Krische, Michael J. (2007-12-01). "Catalytic C−C Coupling via Transfer Hydrogenation: Reverse Prenylation, Crotylation, and Allylation from the Alcohol or Aldehyde Oxidation Level". Journal of the American Chemical Society. 129 (49): 15134–15135. doi:10.1021/ja077389b.
- Ngai, Ming-Yu; Skucas, Eduardas; Krische, Michael J. (2008-07-03). "Ruthenium Catalyzed C−C Bond Formation via Transfer Hydrogenation: Branch-Selective Reductive Coupling of Allenes to Paraformaldehyde and Higher Aldehydes". Organic Letters. 10 (13): 2705–2708. doi:10.1021/ol800836v. PMC 2845390. PMID 18533665.
- Bower, John F.; Patman, Ryan L.; Krische, Michael J. (2008-03-01). "Iridium-Catalyzed C−C Coupling via Transfer Hydrogenation: Carbonyl Addition from the Alcohol or Aldehyde Oxidation Level Employing 1,3-Cyclohexadiene". Organic Letters. 10 (5): 1033–1035. doi:10.1021/ol800159w. PMC 2860772. PMID 18254642.
- Shibahara, Fumitoshi; Bower, John F.; Krische, Michael J. (2008-05-01). "Ruthenium-Catalyzed C−C Bond Forming Transfer Hydrogenation: Carbonyl Allylation from the Alcohol or Aldehyde Oxidation Level Employing Acyclic 1,3-Dienes as Surrogates to Preformed Allyl Metal Reagents". Journal of the American Chemical Society. 130 (20): 6338–6339. doi:10.1021/ja801213x. PMC 2842574. PMID 18444617.
- Kim, In Su; Ngai, Ming-Yu; Krische, Michael J. (2008-05-01). "Enantioselective Iridium-Catalyzed Carbonyl Allylation from the Alcohol or Aldehyde Oxidation Level Using Allyl Acetate as an Allyl Metal Surrogate". Journal of the American Chemical Society. 130 (20): 6340–6341. doi:10.1021/ja802001b. PMC 2858451. PMID 18444616.
- Ketcham, John M.; Shin, Inji; Montgomery, T. Patrick; Krische, Michael J. (2014-08-25). "Catalytic Enantioselective C−H Functionalization of Alcohols by Redox-Triggered Carbonyl Addition: Borrowing Hydrogen, Returning Carbon". Angewandte Chemie International Edition. 53 (35): 9142–9150. doi:10.1002/anie.201403873. PMC 4150357. PMID 25056771.
- Han, Soo Bong; Han, Hoon; Krische, Michael J. (2010-02-17). "Diastereo- and Enantioselective anti-Alkoxyallylation Employing Allylic gem-Dicarboxylates as Allyl Donors via Iridium-Catalyzed Transfer Hydrogenation". Journal of the American Chemical Society. 132 (6): 1760–1761. doi:10.1021/ja9097675. PMC 2824430. PMID 20099821.
- Zhang, Yong Jian; Yang, Jin Haek; Kim, Sang Hoon; Krische, Michael J. (2010-04-07). "anti-Diastereo- and Enantioselective Carbonyl (Hydroxymethyl)allylation from the Alcohol or Aldehyde Oxidation Level: Allyl Carbonates as Allylmetal Surrogates". Journal of the American Chemical Society. 132 (13): 4562–4563. doi:10.1021/ja100949e. PMC 2848290. PMID 20225853.
- Gao, Xin; Zhang, Yong Jian; Krische, Michael J. (2011-04-26). "Iridium-Catalyzed anti-Diastereo- and Enantioselective Carbonyl (α-Trifluoromethyl)allylation from the Alcohol or Aldehyde Oxidation Level". Angewandte Chemie International Edition. 50 (18): 4173–4175. doi:10.1002/anie.201008296. PMC 3161446. PMID 21472938.
- Han, Soo Bong; Gao, Xin; Krische, Michael J. (2010-07-07). "Iridium-Catalyzed anti-Diastereo- and Enantioselective Carbonyl (Trimethylsilyl)allylation from the Alcohol or Aldehyde Oxidation Level". Journal of the American Chemical Society. 132 (26): 9153–9156. doi:10.1021/ja103299f. PMC 2904607. PMID 20540509.
- Hassan, Abbas; Zbieg, Jason R.; Krische, Michael J. (2011-04-04). "Enantioselective Iridium-Catalyzed Vinylogous Reformatsky-Aldol Reaction from the Alcohol Oxidation Level: Linear Regioselectivity by Way of Carbon-Bound Enolates". Angewandte Chemie International Edition. 50 (15): 3493–3496. doi:10.1002/anie.201100646. PMC 3162040. PMID 21381171.
- Han, Soo Bong; Hassan, Abbas; Kim, In Su; Krische, Michael J. (2010-11-10). "Total Synthesis of (+)-Roxaticin via C−C Bond Forming Transfer Hydrogenation: A Departure from Stoichiometric Chiral Reagents, Auxiliaries, and Premetalated Nucleophiles in Polyketide Construction". Journal of the American Chemical Society. 132 (44): 15559–15561. doi:10.1021/ja1082798. PMC 2975273. PMID 20961111.
- Han, Soo Bong; Hassan, Abbas; Kim, In Su; Krische, Michael J. (2010-11-10). "Total Synthesis of (+)-Roxaticin via C−C Bond Forming Transfer Hydrogenation: A Departure from Stoichiometric Chiral Reagents, Auxiliaries, and Premetalated Nucleophiles in Polyketide Construction". Journal of the American Chemical Society. 132 (44): 15559–15561. doi:10.1021/ja1082798. PMC 2975273. PMID 20961111.
- Evans, David A.; Connell, Brian T. (2003-09-01). "Synthesis of the Antifungal Macrolide Antibiotic (+)-Roxaticin". Journal of the American Chemical Society. 125 (36): 10899–10905. doi:10.1021/ja027638q. PMID 12952470.
- Mori, Yuji; Asai, Motoya; Kawade, Jun-ichiro; Okumura, Akiko; Furukawa, Hiroshi (1994). "Total synthesis of the polyene macrolide roxaticin". Tetrahedron Letters. 35 (35): 6503–6506. doi:10.1016/s0040-4039(00)78257-8.
- Mori, Yuji; Asai, Motoya; Kawade, Jun-ichiro; Furukawa, Hiroshi (1995). "Total synthesis of the polyene macrolide antibiotic roxaticin. II. Total synthesis of roxaticin". Tetrahedron. 51 (18): 5315–5330. doi:10.1016/0040-4020(95)00215-t.
- Rychnovsky, Scott D.; Hoye, Rebecca C. (1994-03-01). "Convergent Synthesis of the Polyene Macrolide (-)-Roxaticin". Journal of the American Chemical Society. 116 (5): 1753–1765. doi:10.1021/ja00084a017.
- Lu, Yu; Woo, Sang Kook; Krische, Michael J. (2011-09-07). "Total Synthesis of Bryostatin 7 via C–C Bond-Forming Hydrogenation". Journal of the American Chemical Society. 133 (35): 13876–13879. doi:10.1021/ja205673e. PMC 3164899. PMID 21780806.
- Manaviazar, Soraya; Frigerio, Mark; Bhatia, Gurpreet S.; Hummersone, Marc G.; Aliev, Abil E.; Hale, Karl J. (2006-09-01). "Enantioselective Formal Total Synthesis of the Antitumor Macrolide Bryostatin 7". Organic Letters. 8 (20): 4477–4480. doi:10.1021/ol061626i. PMID 16986929.
- Kageyama, Masanori; Tamura, Tadashi; Nantz, Michael H.; Roberts, John C.; Somfai, Peter; Whritenour, David C.; Masamune, Satoru (1990-09-01). "Synthesis of bryostatin 7". Journal of the American Chemical Society. 112 (20): 7407–7408. doi:10.1021/ja00176a058.
- Feng, Yu; Jiang, Xin; De Brabander, Jef K. (2012-10-17). "Studies toward the Unique Pederin Family Member Psymberin: Full Structure Elucidation, Two Alternative Total Syntheses, and Analogs". Journal of the American Chemical Society. 134 (41): 17083–17093. doi:10.1021/ja3057612. PMC 3482988. PMID 23004238.
- Zhou, Jingjing; Gao, Bowen; Xu, Zhengshuang; Ye, Tao (2016-06-08). "Total Synthesis and Stereochemical Assignment of Callyspongiolide". Journal of the American Chemical Society. 138 (22): 6948–6951. doi:10.1021/jacs.6b03533. PMID 27227371.