Allenes
Allenes are organic compounds in which one carbon atom has double bonds with each of its two adjacent carbon centres.[1] Allenes are classified as cumulated dienes. The parent compound of this class is propadiene, which is itself also called allene. Compounds with an allene-type structure but with more than three carbon atoms are members of a larger class of compounds called cumulenes with X=C=Y bonding. Unlike polyenes, the two double bonds do not exhibit resonance.
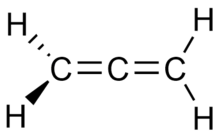
Structure and bonding
Geometry
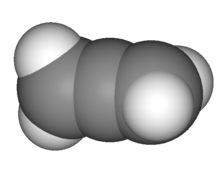
The central carbon atom of allenes forms two sigma bonds and two pi bonds. The central carbon is sp-hybridized, and the two terminal carbon atoms are sp2-hybridized. The bond angle formed by the three carbon atoms is 180°, indicating linear geometry for the central carbon atom. The two terminal carbon atoms are planar, and these planes are twisted 90° from each other. The structure can also be viewed as an "extended tetrahedral" with a similar shape to methane, an analogy that is continued into the stereochemical analysis of certain derivative molecules.
Symmetry

The symmetry and isomerism of allenes has long fascinated organic chemists.[2] For allenes with four identical substituents, there exist two twofold axes of rotation through the center carbon, inclined at 45° to the CH2 planes at either end of the molecule. The molecule can thus be thought of as a two-bladed propeller. A third twofold axis of rotation passes through the C=C=C bonds, and there is a mirror plane passing through both CH2 planes. Thus this class of molecules belong to the D2d point group. Because of the symmetry, an unsubstituted allene has no net dipole moment.
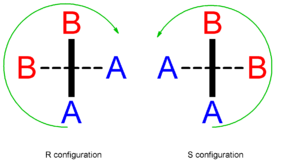
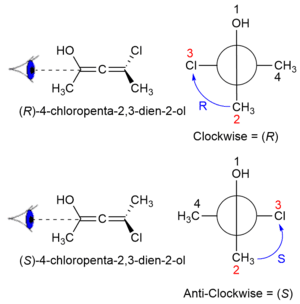
An allene with two different substituents on each of the two carbon atoms will be chiral because there will no longer be any mirror planes. The chirality of these types of allenes was first predicted in 1875 by van 't Hof, but not proven experimentally until 1935.[3] Where A has a greater priority than B according to the Cahn–Ingold–Prelog priority rules, the configuration of the axial chirality can be determined by considering the substituents on the front atom followed by the back atom when viewed along the allene axis. For the back atom, only the group of higher priority need be considered.
Chiral allenes have been recently used as building blocks in the construction of organic materials with exceptional chiroptical properties.[4]
Synthesis
Although allenes often require specialized syntheses, the parent, propadiene is produced on a large scale as an equilibrium mixture with methylacetylene:
- H2C=C=CH2 ⇌ CH3C≡CH
This mixture, known as MAPP gas, is commercially available.
Laboratory methods for the formation of allenes include:
- from geminal dihalocyclopropanes and organolithium compounds (or metallic sodium or magnesium) in the Skattebøl rearrangement (Doering–LaFlamme allene synthesis) via rearrangement of cyclopropylidene carbenes/carbenoids
- from reaction of certain terminal alkynes with formaldehyde, copper(I) bromide, and added base (Crabbé–Ma allene synthesis)[5] [6]
- from dehydrohalogenation of certain dihalides[7]
- from reaction of a triphenylphosphinyl ester with an acid halide, a Wittig reaction accompanied by dehydrohalogenation[8][9]
- from propargylic alcohols via the Myers allene synthesis protocol—a stereospecific process
Occurrence
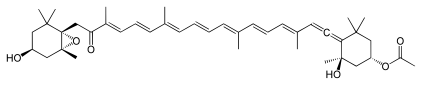
Numerous natural products contain the allene functional group. Noteworthy are the pigments fucoxanthin and peridinin. Little is known about the biosynthesis, although it is conjectured that they are often generated from alkyne precursors.[10]
Allenes serve as ligands in organometallic chemistry. A typical complex is Pt(η2-allene)(PPh3)2. Ni(0) reagents catalyze the cyclooligomerization of allene.[11] Using a suitable catalyst (e.g. Wilkinson's catalyst), it is possible to reduce just one of the double bonds of an allene.[12]
Delta convention
Many rings or ring systems are known by semisystematic names that assume a maximum number of noncumulative bonds. To unambiguously specify derivatives that include cumulated bonds (and hence fewer hydrogens than would be expected from the skeleton), a lowercase delta may be used with a subscript indicating the number of cumulated double bonds from that atom, e.g. 8δ2-Benzocyclononene. This may be combined with the λ-convention for specifying nonstandard valency states, e.g. 2λ4δ2,5λ4δ2-Thieno[3,4-c]thiophene.[13]
See also
- Compounds with three or more adjacent carbon–carbon double bonds are called cumulenes.
References
- IUPAC, Compendium of Chemical Terminology, 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) "allenes". doi:10.1351/goldbook.A00238
- Smith, Michael B.; March, Jerry (2007), Advanced Organic Chemistry: Reactions, Mechanisms, and Structure (6th ed.), New York: Wiley-Interscience, ISBN 978-0-471-72091-1
- Maitland, Peter; Mills, W. H. (1935). "Experimental Demonstration of the Allene Asymmetry". Nature. 135: 994. doi:10.1038/135994a0.
- Rivera Fuentes, Pablo; Diederich, François (2012). "Allenes in Molecular Materials". Angew. Chem. Int. Ed. Engl. 51 (12): 2818–2828. doi:10.1002/anie.201108001. PMID 22308109.
- Crabbé, Pierre; Nassim, Bahman; Robert-Lopes, Maria-Teresa. "One-Step Homologation of Acetylenes to Allenes: 4-Hydroxynona-1,2-diene [1,2-Nonadien-4-ol]". Organic Syntheses. 63: 203. doi:10.15227/orgsyn.063.0203.; Collective Volume, 7, p. 276
- Luo, Hongwen; Ma, Dengke; Ma, Shengming (2017). "Buta-2,3-dien-1-ol". Organic Syntheses: 153–166. doi:10.15227/orgsyn.094.0153.
- Cripps, H. N.; Kiefer, E. F. "Allene". Organic Syntheses. 42: 12. doi:10.15227/orgsyn.042.0012.; Collective Volume, 5, p. 22
- Lang, Robert W.; Hansen, Hans-Jürgen (1980). "Eine einfache Allencarbonsäureester-Synthese mittels der Wittig-Reaktion" [A simple synthesis of allene carboxylic acid esters by means of the Wittig reaction]. Helv. Chim. Acta. 63 (2): 438–455. doi:10.1002/hlca.19800630215.
- Lang, Robert W.; Hansen, Hans-Jürgen. "α-Allenic Esters from α-Phosphoranylidene Esters and Acid Chlorides: Ethyl 2,3-Pentadienoate [2,3-Pentadienoic acid, ethyl ester]". Organic Syntheses. 62: 202. doi:10.15227/orgsyn.062.0202.; Collective Volume, 7, p. 232
- Krause, Norbert; Hoffmann‐Röder, Anja (2004). "18. Allenic Natural Products and Pharmaceuticals". In Krause, Norbert; Hashmi, A. Stephen K. (eds.). Modern Allene Chemistry. doi:10.1002/9783527619573.ch18.
- Otsuka, Sei; Nakamura, Akira "Acetylene and allene complexes: their implication in homogeneous catalysis" Advances in Organometallic Chemistry 1976, volume 14, pp. 245-83. doi:10.1016/S0065-3055(08)60654-1.
- Bhagwat, M. M.; Devaprabhakara, D. (1972). "Selective hydrogenation of allenes with chlorotris-(triphenylphosphine) rhodium catalyst". Tetrahedron Letters. 13 (15): 1391–1392. doi:10.1016/S0040-4039(01)84636-0.
- "Nomenclature for cyclic organic compounds with contiguous formal double bonds (the δ-convention)". Pure Appl. Chem. 60: 1395-1401. 1988. doi:10.1351/pac198860091395.
Further reading
- Brummond, Kay M. (editor). Allene chemistry (special thematic issue). Beilstein Journal of Organic Chemistry 7: 394–943.