Iron(II) lactate
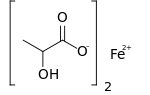
Ferrous lactate, or iron(II) lactate, is a chemical compound consisting of one atom of iron (Fe2+) and two lactate anions. It has the chemical formula Fe(C3H5O3)2.
 | |
| Names | |
|---|---|
| IUPAC name
Ferrous 2-hydroxypropanoate | |
| Other names
Iron dilactate Iron(II) lactate E585 | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.025.098 |
| E number | E585 (acidity regulators, ...) |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C6H10FeO6 | |
| Molar mass | 265.983 g/mol |
| Appearance | greenish-white powder |
| Melting point | 500 °C (932 °F; 773 K) |
| trihydrate: 2.1 g/100ml (10 °C) 8.5 g/100ml (100 °C) dihydrate: 2% (25 °C)[1] | |
| Solubility | soluble in alkali citrates negligible in alcohol insoluble in ether |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Uses
It is used as a food additive with E number E585. It is an acidity regulator and colour retention agent, and is also used to fortify foods with iron.
Safety
It is toxic and may cause irritation. Avoid inhalation of dusts. Remove all contamination, rinse with plenty of water. May cause some health symptoms including nausea after ingestion both acute and delayed.[1]
References
- Iron(II) lactate dihydrate MSDS Archived 2014-05-03 at the Wayback Machine at Jost Chemical
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.