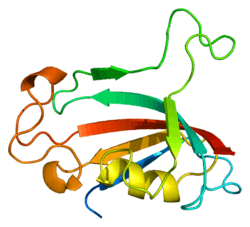
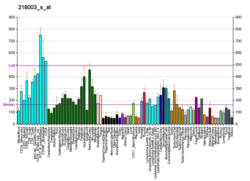
FKBP3
FK506-binding protein 3 is a protein that in humans is encoded by the FKBP3 gene.[5][6][7]
Function
The protein encoded by this gene is a member of the immunophilin protein family, which play a role in immunoregulation and basic cellular processes involving protein folding and trafficking. This encoded protein is a cis-trans prolyl isomerase that binds the immunosuppressants FK506 and rapamycin. It has a higher affinity for rapamycin than for FK506 and thus may be an important target molecule for immunosuppression by rapamycin.[7]
Interactions
FKBP3 has been shown to interact with YY1,[8] HDAC1,[8] Histone deacetylase 2,[8] DNA,[9] and Mdm2.[10]
References
- GRCh38: Ensembl release 89: ENSG00000100442 - Ensembl, May 2017
- GRCm38: Ensembl release 89: ENSMUSG00000020949 - Ensembl, May 2017
- "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- Hung DT, Schreiber SL (Jun 1992). "cDNA cloning of a human 25 kDa FK506 and rapamycin binding protein". Biochem. Biophys. Res. Commun. 184 (2): 733–8. doi:10.1016/0006-291X(92)90651-Z. PMID 1374240.
- Porat R, Poutsiaka DD, Miller LC, Granowitz EV, Dinarello CA (May 1992). "Interleukin-1 (IL-1) receptor blockade reduces endotoxin and Borrelia burgdorferi-stimulated IL-8 synthesis in human mononuclear cells". FASEB J. 6 (7): 2482–6. PMID 1532945.
- "Entrez Gene: FKBP3 FK506 binding protein 3, 25kDa".
- Yang WM, Yao YL, Seto E (Sep 2001). "The FK506-binding protein 25 functionally associates with histone deacetylases and with transcription factor YY1". EMBO J. 20 (17): 4814–25. doi:10.1093/emboj/20.17.4814. PMC 125595. PMID 11532945.
- Prakash A, Shin J, Rajan S, Yoon HS (2016). "Structural basis of nucleic acid recognition by FK506-binding protein 25 (FKBP25), a nuclear immunophilin". Nucleic Acids Res. 44 (6): 2909–25. doi:10.1093/nar/gkw001. PMC 4824100. PMID 26762975.
- Ochocka AM, Kampanis P, Nicol S, Allende-Vega N, Cox M, Marcar L, Milne D, Fuller-Pace F, Meek D (Feb 2009). "FKBP25, a novel regulator of the p53 pathway, induces the degradation of MDM2 and activation of p53". FEBS Lett. 583 (4): 621–6. doi:10.1016/j.febslet.2009.01.009. PMID 19166840.
Further reading
- Jin YJ, Burakoff SJ, Bierer BE (1992). "Molecular cloning of a 25-kDa high affinity rapamycin binding protein, FKBP25". J. Biol. Chem. 267 (16): 10942–5. PMID 1375932.
- Wiederrecht G, Martin MM, Sigal NH, Siekierka JJ (1992). "Isolation of a human cDNA encoding a 25 kDa FK-506 and rapamycin binding protein". Biochem. Biophys. Res. Commun. 185 (1): 298–303. doi:10.1016/S0006-291X(05)80990-8. PMID 1376117.
- Jin YJ, Burakoff SJ (1993). "The 25-kDa FK506-binding protein is localized in the nucleus and associates with casein kinase II and nucleolin". Proc. Natl. Acad. Sci. U.S.A. 90 (16): 7769–73. doi:10.1073/pnas.90.16.7769. PMC 47224. PMID 7689229.
- Cross SH, Charlton JA, Nan X, Bird AP (1994). "Purification of CpG islands using a methylated DNA binding column". Nat. Genet. 6 (3): 236–44. doi:10.1038/ng0394-236. PMID 8012384.
- Maruyama K, Sugano S (1994). "Oligo-capping: a simple method to replace the cap structure of eukaryotic mRNAs with oligoribonucleotides". Gene. 138 (1–2): 171–4. doi:10.1016/0378-1119(94)90802-8. PMID 8125298.
- Suzuki Y, Yoshitomo-Nakagawa K, Maruyama K, Suyama A, Sugano S (1997). "Construction and characterization of a full length-enriched and a 5'-end-enriched cDNA library". Gene. 200 (1–2): 149–56. doi:10.1016/S0378-1119(97)00411-3. PMID 9373149.
- Meng X, Chen J, Yang Q, Wang S, Chao Y, Ying K, Xie Y, Mao Y (2002). "Cloning and identification of a novel cDNA which may be associated with FKBP25". Biochem. Genet. 40 (9–10): 303–10. doi:10.1023/A:1020256718720. PMID 12392168.
- Rual JF, Venkatesan K, Hao T, Hirozane-Kishikawa T, Dricot A, Li N, Berriz GF, Gibbons FD, Dreze M, Ayivi-Guedehoussou N, Klitgord N, Simon C, Boxem M, Milstein S, Rosenberg J, Goldberg DS, Zhang LV, Wong SL, Franklin G, Li S, Albala JS, Lim J, Fraughton C, Llamosas E, Cevik S, Bex C, Lamesch P, Sikorski RS, Vandenhaute J, Zoghbi HY, Smolyar A, Bosak S, Sequerra R, Doucette-Stamm L, Cusick ME, Hill DE, Roth FP, Vidal M (2005). "Towards a proteome-scale map of the human protein-protein interaction network". Nature. 437 (7062): 1173–8. doi:10.1038/nature04209. PMID 16189514.
External links
- PDBe-KB provides an overview of all the structure information available in the PDB for Human Peptidyl-prolyl cis-trans isomerase FKBP3
- PDBe-KB provides an overview of all the structure information available in the PDB for Mouse Peptidyl-prolyl cis-trans isomerase FKBP3
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.