Elastomer
An Elastomer is a polymer with viscoelasticity (i.e., both viscosity and elasticity) and has very weak intermolecular forces, generally low Young's modulus and high failure strain compared with other materials.[1] The term, a portmanteau of elastic polymer,[2] is often used interchangeably with rubber, although the latter is preferred when referring to vulcanisates.[3] Each of the monomers which link to form the polymer is usually a compound of several elements among carbon, hydrogen, oxygen and silicon. Elastomers are amorphous polymers maintained above their glass transition temperature, so that considerable molecular reconformation, without breaking of covalent bonds, is feasible. At ambient temperatures, such rubbers are thus relatively compliant (E ≈ 3 MPa) and deformable. Their primary uses are for seals, adhesives and molded flexible parts. Application areas for different types of rubber are manifold and cover segments as diverse as tires, soles for shoes, and damping and insulating elements. The importance of these rubbers can be judged from the fact that global revenues are forecast to rise to US$56 billion in 2020.[4][5]
IUPAC defines the term "elastomer" by "Polymer that displays rubber-like elasticity."[6]
Manufacturers of Elastomeric parts include NoProto, PrintForm, 3D Systems, and Afformativ.
Rubber-like solids with elastic properties are called elastomers. Polymer chains are held together in these materials by relatively weak intermolecular bonds, which permit the polymers to stretch in response to macroscopic stresses. Natural rubber, neoprene rubber, buna-s and buna-n are all examples of such elastomers.
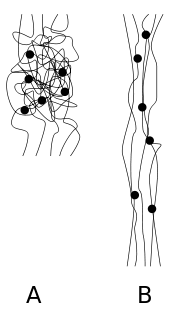
Elastomers are usually thermosets (requiring vulcanization) but may also be thermoplastic (see thermoplastic elastomer). The long polymer chains cross-link during curing, i.e., vulcanizing. The molecular structure of elastomers can be imagined as a 'spaghetti and meatball' structure, with the meatballs signifying cross-links. The elasticity is derived from the ability of the long chains to reconfigure themselves to distribute an applied stress. The covalent cross-linkages ensure that the elastomer will return to its original configuration when the stress is removed. As a result of this extreme flexibility, elastomers can reversibly extend from 5–700%, depending on the specific material. Without the cross-linkages or with short, uneasily reconfigured chains, the applied stress would result in a permanent deformation.
Temperature effects are also present in the demonstrated elasticity of a polymer. Elastomers that have cooled to a glassy or crystalline phase will have less mobile chains, and consequentially less elasticity, than those manipulated at temperatures higher than the glass transition temperature of the polymer.
It is also possible for a polymer to exhibit elasticity that is not due to covalent cross-links, but instead for thermodynamic reasons.
Examples
Unsaturated rubbers that can be cured by sulfur vulcanization:
- Natural polyisoprene: cis-1,4-polyisoprene natural rubber (NR) and trans-1,4-polyisoprene gutta-percha
- Synthetic polyisoprene (IR for isoprene rubber)
- Polybutadiene (BR for butadiene rubber)
- Chloroprene rubber (CR), polychloroprene, Neoprene, Baypren etc.
- Butyl rubber (copolymer of isobutylene and isoprene, IIR)
- Halogenated butyl rubbers (chloro butyl rubber: CIIR; bromo butyl rubber: BIIR)
- Styrene-butadiene Rubber (copolymer of styrene and butadiene, SBR)
- Nitrile rubber (copolymer of butadiene and acrylonitrile, NBR), also called Buna N rubbers
- Hydrogenated Nitrile Rubbers (HNBR) Therban and Zetpol
(Unsaturated rubbers can also be cured by non-sulfur vulcanization if desired.)
Saturated rubbers that cannot be cured by sulfur vulcanization:
- EPM (ethylene propylene rubber, a copolymer of ethylene and propylene) and EPDM rubber (ethylene propylene diene rubber, a terpolymer of ethylene, propylene and a diene-component)
- Epichlorohydrin rubber (ECO)
- Polyacrylic rubber (ACM, ABR)
- Silicone rubber (SI, Q, VMQ)
- Fluorosilicone Rubber (FVMQ)
- Fluoroelastomers (FKM, and FEPM) Viton, Tecnoflon, Fluorel, Aflas and Dai-El
- Perfluoroelastomers (FFKM) Tecnoflon PFR, Kalrez, Chemraz, Perlast
- Polyether block amides (PEBA)
- Chlorosulfonated polyethylene (CSM), (Hypalon)
- Ethylene-vinyl acetate (EVA)
"The definitions are not authentic as the Rubber which is classified in World Customs Organisation Books in Chapter 40, where as the above definitions stating all rubber and different polymers in same chapter which is classified in Chapter 39 of the World Custom Organisation's Harmonised Commodity for Description and coding system. One should go through all differentiation while editing between Plastics and articles thereof and Rubber and articles thereof."
Various other types of 4S elastomers:
- Thermoplastic elastomers (TPE)
- The proteins resilin and elastin
- Polysulfide rubber
- Elastolefin, elastic fiber used in fabric production
See also
References
- De, Sadhan K. (31 December 1996). Rubber Technologist's Handbook, Volume 1 (1st ed.). Smithers Rapra Press. p. 287. ISBN 978-1859572627. Archived from the original on 2017-02-07. Retrieved 7 February 2017.
- Gent, Alan N. "Elastomer Chemical Compound". Encyclopædia Britannica. Encyclopædia Britannica. Archived from the original on 2017-02-07. Retrieved 7 February 2017.
- Alger, Mark (21 April 1989). Polymer Science Dictionary. Springer. p. 503. ISBN 1851662200. Archived from the original on 2017-02-07. Retrieved 7 February 2017.
- "Market Study on Synthetic Rubber". Ceresana.com. Archived from the original on 2013-06-29. Retrieved 2013-06-28.
- "Global rubber market to generate $56 billion by 2020". British Plastics and Rubber (BP&R). Archived from the original on 2018-09-22. Retrieved 2018-09-21.
- "Definitions of terms relating to the structure and processing of sols, gels, networks, and inorganic–organic hybrid materials (IUPAC Recommendations 2007)" (PDF). Pure and Applied Chemistry. 79 (10): 1801–1829. 2007. doi:10.1351/pac200779101801. Archived (PDF) from the original on 2018-01-06. Retrieved 2017-07-14.