Drude model
The Drude model of electrical conduction was proposed in 1900[1][2] by Paul Drude to explain the transport properties of electrons in materials (especially metals). The model, which is an application of kinetic theory, assumes that the microscopic behaviour of electrons in a solid may be treated classically and looks much like a pinball machine, with a sea of constantly jittering electrons bouncing and re-bouncing off heavier, relatively immobile positive ions.

The two most significant results of the Drude model are an electronic equation of motion,
and a linear relationship between current density J and electric field E,
Here t is the time, ⟨p⟩ is the average momentum per electron and q, n, m, and τ are respectively the electron charge, number density, mass, and mean free time between ionic collisions (that is, the mean time an electron has traveled since the last collision). The latter expression is particularly important because it explains in semi-quantitative terms why Ohm's law, one of the most ubiquitous relationships in all of electromagnetism, should be true.[note 1][3][4]
The model was extended in 1905 by Hendrik Antoon Lorentz (and hence is also known as the Drude–Lorentz model) to give the relation between the thermal conductivity and the electric conductivity of metals (see Lorenz number), and is a classical model. Later it was supplemented with the results of quantum theory in 1933 by Arnold Sommerfeld and Hans Bethe, leading to the Drude–Sommerfeld model.
Assumptions
The Drude model considers the metal to be formed of a mass of positively charged ions from which a number of "free electrons" were detached. These may be thought to have become delocalized when the valence levels of the atom came in contact with the potential of the other atoms.[note 2]
The Drude model neglects any long-range interaction between the electron and the ions or between the electrons. The only possible interaction of a free electron with its environment is via instantaneous collisions. The average time between subsequent collisions of such an electron is τ, and the nature of the collision partner of the electron does not matter for the calculations and conclusions of the Drude model.[note 2]
After a collision event, the velocity (and direction) of the electron only depends on the local temperature distribution and is completely independent of the velocity of the electron before the collision event.[note 2]
Explanations
DC field
The simplest analysis of the Drude model assumes that electric field E is both uniform and constant, and that the thermal velocity of electrons is sufficiently high such that they accumulate only an infinitesimal amount of momentum dp between collisions, which occur on average every τ seconds.[note 1]
Then an electron isolated at time t will on average have been travelling for time τ since its last collision, and consequently will have accumulated momentum
During its last collision, this electron will have been just as likely to have bounced forward as backward, so all prior contributions to the electron's momentum may be ignored, resulting in the expression
Substituting the relations
results in the formulation of Ohm's law mentioned above:
Time-varying analysis
The dynamics may also be described by introducing an effective drag force. At time t = t0 + dt the average electron's momentum will be
because, on average, a fraction of 1 − dt/τ of the electrons will not have experienced another collision, and the ones that have will contribute to the total momentum to only a negligible order.[note 3]
With a bit of algebra and dropping terms of order dt2, this results in the differential equation
where ⟨p⟩ denotes average momentum and q the charge of the electrons. This, which is an inhomogeneous differential equation, may be solved to obtain the general solution of
for p(t). The steady state solution, d ⟨p⟩/dt = 0, is then
As above, average momentum may be related to average velocity and this in turn may be related to current density,
and the material can be shown to satisfy Ohm's law with a DC-conductivity σ0:
AC field
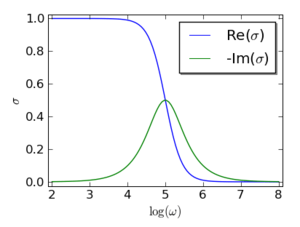
The Drude model can also predict the current as a response to a time-dependent electric field with an angular frequency ω. The complex conductivity is
Here it is assumed that:
In engineering, i is generally replaced by −i (or −j ) in all equations, which reflects the phase difference with respect to origin, rather than delay at the observation point traveling in time. The imaginary part indicates that the current lags behind the electrical field. This happens because the electrons need roughly a time τ to accelerate in response to a change in the electrical field. Here the Drude model is applied to electrons; it can be applied both to electrons and holes; i.e., positive charge carriers in semiconductors. The curves for σ(ω) are shown in the graph.
If a sinusoidally varying electric field with frequency is applied to the solid, the negatively charged electrons behave as a plasma that tends to move a distance x apart from the positively charged background. As a result, the sample is polarized and there will be an excess charge at the opposite surfaces of the sample.
The dielectric constant of the sample is expressed as
where is the electric displacement and is the polarization density.
The polarization density is written as
and the polarization density with n electron density is
After a little algebra the relation between polarization density and electric field can be expressed as
The frequency dependent dielectric function of the solid is
At a resonance frequency , called the plasma frequency, the dielectric function changes sign from negative to positive and real part of the dielectric function drops to zero.
The plasma frequency represents a plasma oscillation resonance or plasmon. The plasma frequency can be employed as a direct measure of the square root of the density of valence electrons in a solid. Observed values are in reasonable agreement with this theoretical prediction for a large number of materials.[5] Below the plasma frequency, the dielectric function is negative and the field cannot penetrate the sample. Light with angular frequency below the plasma frequency will be totally reflected. Above the plasma frequency the light waves can penetrate the sample.
Drude response in real materials
The characteristic behavior of a Drude metal in the time or frequency domain, i.e. exponential relaxation with time constant τ or the frequency dependence for σ(ω) stated above, is called Drude response. In a conventional, simple, real metal (e.g. sodium, silver, or gold at room temperature) such behavior is not found experimentally, because the characteristic frequency τ−1 is in the infrared frequency range, where other features that are not considered in the Drude model (such as band structure) play an important role.[6] But for certain other materials with metallic properties, frequency-dependent conductivity was found that closely follows the simple Drude prediction for σ(ω). These are materials where the relaxation rate τ−1 is at much lower frequencies.[6] This is the case for certain doped semiconductor single crystals,[7] high-mobility two-dimensional electron gases,[8] and heavy-fermion metals.[9]
Accuracy of the model
Historically, the Drude formula was first derived in a limited way, namely by assuming that the charge carriers form a classical ideal gas. Arnold Sommerfeld considered quantum theory and extended the theory to the free electron model, where the carriers follow Fermi–Dirac distribution. The conductivity predicted is the same as in the Drude model because it does not depend on the form of the electronic speed distribution.
The Drude model provides a very good explanation of DC and AC conductivity in metals, the Hall effect, and the magnetoresistance[note 3] in metals near room temperature. The model also explains partly the Wiedemann–Franz law of 1853. However, it greatly overestimates the electronic heat capacities of metals. In reality, metals and insulators have roughly the same heat capacity at room temperature.
The model can also be applied to positive (hole) charge carriers.
In his original paper, Drude made an error, estimating the Lorenz number of Wiedemann–Franz law to be twice what it classically should have been, thus making it seem in agreement with the experimental value for the specific heat is about 100 times smaller than the classical prediction but this factor cancels out with the mean electronic speed that is about 100 times bigger than Drude's calculation.[note 4]
See also
- Free electron model
- Arnold Sommerfeld
- Electrical conductivity
Notes
- Ashcroft & Mermin 1976, pp. 6–7
- Ashcroft & Mermin 1976, pp. 2–6
- Ashcroft & Mermin 1976, p. 11
- Ashcroft & Mermin 1976, p. 23
References
- Drude, Paul (1900). "Zur Elektronentheorie der Metalle". Annalen der Physik. 306 (3): 566–613. Bibcode:1900AnP...306..566D. doi:10.1002/andp.19003060312.
- Drude, Paul (1900). "Zur Elektronentheorie der Metalle; II. Teil. Galvanomagnetische und thermomagnetische Effecte". Annalen der Physik. 308 (11): 369–402. Bibcode:1900AnP...308..369D. doi:10.1002/andp.19003081102.
- Edward M. Purcell (1965). Electricity and Magnetism. McGraw-Hill. pp. 117–122. ISBN 978-0-07-004908-6.
- David J. Griffiths (1999). Introduction to Electrodynamics. Prentice-Hall. pp. 289. ISBN 978-0-13-805326-0.
- C. Kittel (1953–1976). Introduction to Solid State Physics. Wiley & Sons. ISBN 978-0-471-49024-1.
- M. Dressel; M. Scheffler (2006). "Verifying the Drude response". Annalen der Physik. 15 (7–8): 535–544. Bibcode:2006AnP...518..535D. doi:10.1002/andp.200510198.
- M. van Exter; D. Grischkowsky (1990). "Carrier dynamics of electrons and holes in moderately doped silicon" (PDF). Physical Review B. 41 (17): 12140–12149. Bibcode:1990PhRvB..4112140V. doi:10.1103/PhysRevB.41.12140. hdl:11244/19898.
- P. J. Burke; I. B. Spielman; J. P. Eisenstein; L. N. Pfeiffer; K. W. West (2000). "High frequency conductivity of the high-mobility two-dimensional electron gas" (PDF). Applied Physics Letters. 76 (6): 745–747. Bibcode:2000ApPhL..76..745B. doi:10.1063/1.125881.
- M. Scheffler; M. Dressel; M. Jourdan; H. Adrian (2005). "Extremely slow Drude relaxation of correlated electrons". Nature. 438 (7071): 1135–1137. Bibcode:2005Natur.438.1135S. doi:10.1038/nature04232. PMID 16372004.
General
- Ashcroft, Neil; Mermin, N. David (1976). Solid State Physics. New York: Holt, Rinehart and Winston. ISBN 978-0-03-083993-1.CS1 maint: ref=harv (link)
External links
- Heaney, Michael B (2003). "Electrical Conductivity and Resistivity". In Webster, John G. (ed.). Electrical Measurement, Signal Processing, and Displays. CRC Press. ISBN 9780203009406.