Carbonic anhydrase
The carbonic anhydrases (or carbonate dehydratases) form a family of enzymes that catalyze the interconversion between carbon dioxide and water and the dissociated ions of carbonic acid (i.e. bicarbonate and hydrogen ions).[1] The active site of most carbonic anhydrases contains a zinc ion. They are therefore classified as metalloenzymes. The enzyme maintains acid-base balance and helps transport carbon dioxide.[2]
| Carbonate dehydratase | |||||||||
|---|---|---|---|---|---|---|---|---|---|
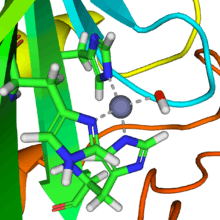
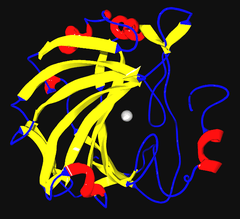 Ribbon diagram of human carbonic anhydrase II, with zinc ion visible in the center | |||||||||
| Identifiers | |||||||||
| EC number | 4.2.1.1 | ||||||||
| CAS number | 9001-03-0 | ||||||||
| Databases | |||||||||
| IntEnz | IntEnz view | ||||||||
| BRENDA | BRENDA entry | ||||||||
| ExPASy | NiceZyme view | ||||||||
| KEGG | KEGG entry | ||||||||
| MetaCyc | metabolic pathway | ||||||||
| PRIAM | profile | ||||||||
| PDB structures | RCSB PDB PDBe PDBsum | ||||||||
| Gene Ontology | AmiGO / QuickGO | ||||||||
| |||||||||
| Eukaryotic-type carbonic anhydrase | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Identifiers | |||||||||
| Symbol | Carb_anhydrase | ||||||||
| Pfam | PF00194 | ||||||||
| InterPro | IPR001148 | ||||||||
| PROSITE | PDOC00146 | ||||||||
| SCOPe | 1can / SUPFAM | ||||||||
| Membranome | 333 | ||||||||
| |||||||||
Carbonic anhydrase helps regulate pH and fluid balance. Depending on its location, the role of the enzyme changes slightly. For example, carbonic anhydrase produces acid in the stomach lining. In the kidney, the control of bicarbonate ions influences the water content of the cell. The control of bicarbonate ions also influences the water content in the eyes. Inhibitors of carbonic anhydrase are used to treat glaucoma, the excessive build up of water in the eyes. Blocking this enzyme shifts the fluid balance in the eyes of the patient to reduce fluid build up thereby relieving pressure.[2][3]
The Bohr Effect is a way to describe hemoglobin's oxygen binding affinity. The Bohr Effect was described by Christian Bohr in the year 1904, and it refers to a shift in an oxygen dissociation curve that is caused by a change in concentration of carbon dioxide or a change in the pH. Essentially an increase in carbon dioxide results in lowered blood pH which lowers oxygen-hemoglobin binding.[4] The opposite is true where a decrease in the concentration of carbon dioxide raises the blood pH which raises the rate of oxygen-hemoglobin binding. Relating the Bohr Effect to carbonic anhydrase is simple: carbonic anhydrase speeds up the reaction of carbon dioxide reacting with water to produce hydrogen protons and bicarbonate ions.
To describe equilibrium in the carbonic anhydrase reaction, Le Chatelier's principle is used. The tissues are more acidic than the lungs because carbon dioxide is produced by cellular respiration and it reacts with water in the tissues to produce the hydrogen protons. Because the carbon dioxide concentration is higher, equilibrium shifts to the right, to the bicarbonate side. The opposite is seen in the lungs where carbon dioxide is being released so its concentration is lower so equilibrium shifts to the left towards carbon dioxide to try and raise its concentration.[5]
Background
An enzyme is known as a substance that acts as a catalyst in living organisms which helps to speed up chemical reactions.[6] Carbonic anhydrase is one important enzyme that is found in red blood cells, gastric mucosa, pancreatic cells, and even renal tubules. It is a very old enzyme that was discovered in the year 1932 and it has been categorized into three general classes.[7] Class one being alpha carbonic anhydrase which is found in mammals, class two being beta carbonic anhydrase which is found in bacteria and plants and lastly, class three which is gamma carbonic anhydrase which is found in methanogen bacteria in hot springs.[8] The three classes of carbonic anhydrase all have the same active site with a Zn metal centre however they are not structurally similar to each other. The main role of carbonic anhydrase in humans is to catalyze the conversion of carbon dioxide to carbonic acid and back again. However, it can also help with CO2 transport in the blood which in turn helps respiration. It can even function in the formation of hydrochloric acid by the stomach.[6] Therefore, the role of carbonic anhydrase depends on where it is found in the body.
Reaction
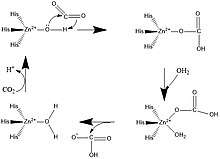
The reaction that shows the catalyzation of carbonic anhydrase in our tissues is: CO2 + H2O H2CO3 H+ + HCO3-. The catalyzation of carbonic anhydrase in the lungs is shown by: H+ + HCO3- H2CO3 CO2 + H2O. The reason for the reactions being in opposite directions for the tissues and lungs is because of the different pH levels found in them. Without the carbonic anhydrase catalyst, the reaction is very slow, however with the catalyst the reaction is 107 times faster.
The reaction catalyzed by carbonic anhydrase is:
- HCO3− + H+ CO2 + H2O
Carbonic acid has a pKa of around 6.36 (the exact value depends on the medium), so at pH 7 a small percentage of the bicarbonate is protonated.
Carbonic anhydrase is one of the fastest enzymes, and its rate is typically limited by the diffusion rate of its substrates. Typical catalytic rates of the different forms of this enzyme ranging between 104 and 106 reactions per second.[9]
The uncatalyzed reverse reaction is relatively slow (kinetics in the 15-second range). This is why a carbonated drink does not instantly degas when opening the container; however, it will rapidly degas in the mouth when it comes in contact with carbonic anhydrase that is contained in saliva.[10]
An anhydrase is defined as an enzyme that catalyzes the removal of a water molecule from a compound, and so it is this "reverse" reaction that gives carbonic anhydrase its name, because it removes a water molecule from carbonic acid.
In the lungs carbonic anhydrase converts bicarbonate to carbon dioxide, suited for exhalation.
Mechanism
A zinc prosthetic group in the enzyme is coordinated in three positions by histidine side-chains. The fourth coordination position is occupied by water. A fourth histidine is close to the water ligand, facilitating formation of Zn-OH center, which binds CO2 to give a zinc bicarbonate.[11] The construct is an example of general acid – general base catalysis (see the article "Acid catalysis"). The active site also features a pocket suited for carbon dioxide, bringing it close to the hydroxide group.
Families

Carbonic anhydrase was initially found in the red blood cells of cows.[2]
At least five distinct CA families are recognized: α, β, γ, δ and ζ. These families have no significant amino acid sequence similarity and in most cases are thought to be an example of convergent evolution. The α-CAs are found in humans.
α-CA
Vertebrates, algae and some bacteria have this family of CAs.
The CA enzymes found in mammals are divided into four broad subgroups,[12] which, in turn consist of several isoforms:
- the cytosolic CAs (CA-I, CA-II, CA-III, CA-VII and CA XIII) (CA1, CA2, CA3, CA7, CA13)
- mitochondrial CAs (CA-VA and CA-VB) (CA5A, CA5B)
- secreted CAs (CA-VI) (CA6)
- membrane-associated CAs (CA-IV, CA-IX, CA-XII, CA-XIV and CA-XV) (CA4, CA9, CA12, CA14)
There are three additional "acatalytic" human carbonic anhydrase isoforms (CA-VIII, CA-X, and CA-XI) (CA8, CA10, CA11) whose functions remain unclear.[13]
| Isoform | Gene | Molecular mass[15] | Location (cell) | Location (tissue)[15] | Specific activity of human enzymes (except for mouse CA XV) (s−1)[16] |
Sensitivity to sulfonamides (acetazolamide in this table) KI (nM)[16] |
|---|---|---|---|---|---|---|
| CA-I | CA1 | 29 kDa | cytosol | red blood cell and GI tract | 2.0 × 105 | 250 |
| CA-II | CA2 | 29 kDa | cytosol | almost ubiquitous | 1.4 × 106 | 12 |
| CA-III | CA3 | 29 kDa | cytosol | 8% of soluble protein in Type I muscle | 1.3 × 104 | 240000 |
| CA-IV | CA4 | 35 kDa | extracellular GPI-linked | GI tract, kidney, endothelium | 1.1 × 106 | 74 |
| CA-VA | CA5A | 34.7 kDa (predicted) | mitochondria | liver | 2.9 × 105 | 63 |
| CA-VB | CA5B | 36.4 kDa (predicted) | mitochondria | widely distributed | 9.5 × 105 | 54 |
| CA-VI | CA6 | 39–42 kDa | secretory | saliva and milk | 3.4 × 105 | 11 |
| CA-VII | CA7 | 29 kDa | cytosol | widely distributed | 9.5 × 105 | 2.5 |
| CA-IX | CA9 | 54, 58 kDa | cell membrane-associated | normal GI tract, several cancers | 1.1 × 106 | 16 |
| CA-XII | CA12 | 44 kDa | extracellularily located active site | kidney, certain cancers | 4.2 × 105 | 5.7 |
| CA-XIII[17] | CA13 | 29 kDa | cytosol | widely distributed | 1.5 × 105 | 16 |
| CA-XIV | CA14 | 54 kDa | extracellularily located active site | kidney, heart, skeletal muscle, brain | 3.1 × 105 | 41 |
| CA-XV[18] | CA15 | 34–36 kDa | extracellular GPI-linked | kidney, not expressed in human tissues | 4.7 × 105 | 72 |
β-CA
Most prokaryotic and plant chloroplast CAs belong to the beta family. Two signature patterns for this family have been identified:
- C-[SA]-D-S-R-[LIVM]-x-[AP]
- [EQ]-[YF]-A-[LIVM]-x(2)-[LIVM]-x(4)-[LIVMF](3)-x-G-H-x(2)-C-G
γ-CA
The gamma class of CAs come from methanogens, methane-producing bacteria that grow in hot springs.
δ-CA
The delta class of CAs has been described in diatoms. The distinction of this class of CA has recently[19] come into question, however.
ζ-CA
The zeta class of CAs occurs exclusively in bacteria in a few chemolithotrophs and marine cyanobacteria that contain cso-carboxysomes.[20] Recent 3-dimensional analyses[19] suggest that ζ-CA bears some structural resemblance to β-CA, particularly near the metal ion site. Thus, the two forms may be distantly related, even though the underlying amino acid sequence has since diverged considerably.
η-CA
The eta family of CAs was recently found in organisms of the genus Plasmodium. These are a group of enzymes previously thought to belong to the alpha family of CAs, however it has been demonstrated that η-CAs have unique features, such as their metal ion coordination pattern.[21]
Structure and function
Several forms of carbonic anhydrase occur in nature. In the best-studied α-carbonic anhydrase form present in animals, the zinc ion is coordinated by the imidazole rings of 3 histidine residues, His94, His96, and His119.
The primary function of the enzyme in animals is to interconvert carbon dioxide and bicarbonate to maintain acid-base balance in blood and other tissues, and to help transport carbon dioxide out of tissues.
There are at least 14 different isoforms in mammals. Plants contain a different form called β-carbonic anhydrase, which, from an evolutionary standpoint, is a distinct enzyme, but participates in the same reaction and also uses a zinc ion in its active site. In plants, carbonic anhydrase helps raise the concentration of CO2 within the chloroplast in order to increase the carboxylation rate of the enzyme RuBisCO. This is the reaction that integrates CO2 into organic carbon sugars during photosynthesis, and can use only the CO2 form of carbon, not carbonic acid or bicarbonate.
Cadmium-containing carbonic anhydrase
Marine diatoms have been found to express a new form of ζ carbonic anhydrase. T. weissflogii, a species of phytoplankton common to many marine ecosystems, was found to contain carbonic anhydrase with a cadmium ion in place of zinc.[22] Previously, it had been believed that cadmium was a toxic metal with no biological function whatsoever. However, this species of phytoplankton appears to have adapted to the low levels of zinc in the ocean by using cadmium when there is not enough zinc.[23] Although the concentration of cadmium in sea water is also low (about 1x10−16 molar), there is an environmental advantage to being able to use either metal depending on which is more available at the time. This type of carbonic anhydrase is therefore cambialistic, meaning it can interchange the metal in its active site with other metals (namely, zinc and cadmium).[24]
Similarities to other carbonic anhydrases
The mechanism of cadmium carbonic anhydrase (CDCA) is essentially the same as that of other carbonic anhydrases in its conversion of carbon dioxide and water into bicarbonate and a proton.[25] Additionally, like the other carbonic anhydrases, CDCA makes the reaction go almost as fast as the diffusion rate of its substrates, and it can be inhibited by sulfonamide and sulfamate derivatives.[25]
Differences from other carbonic anhydrases
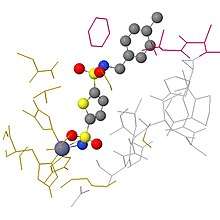
Unlike most other carbonic anhydrases, the active site metal ion is not bound by three histidine residues and a hydroxide ion. Instead, it is bound by two cysteine residues, one histidine residue, and a hydroxide ion, which is characteristic of β-CA.[25][26] Due to the fact that cadmium is a soft acid, it will be more tightly bound by soft base ligands.[24] The sulfur atoms on the cysteine residues are soft bases, thus binding the cadmium more tightly than the nitrogen on histidine residues would. CDCA also has a three-dimensional folding structure that is unlike any other carbonic anhydrase, and its amino acid sequence is dissimilar to the other carbonic anhydrases.[25] It is a monomer with three domains, each one identical in amino acid sequence and each one containing an active site with a metal ion.[26]
Another key difference between CDCA and the other carbonic anhydrases is that CDCA has a mechanism for switching out its cadmium ion for a zinc ion in the event that zinc becomes more available to the phytoplankton than cadmium. The active site of CDCA is essentially "gated" by a chain of nine amino acids with glycine residues at positions 1 and 9. Normally, this gate remains closed and the cadmium ion is trapped inside. However, due to the flexibility and position of the glycine residues, this gate can be opened in order to remove the cadmium ion. A zinc ion can then be put in its place and the gate will close behind it.[25] As a borderline acid, zinc will not bind as tightly to the cysteine ligands as cadmium would, but the enzyme will still be active and reasonably efficient. The metal in the active site can be switched between zinc and cadmium depending on which one is more abundant at the time. It is the ability of CDCA to utilize either cadmium or zinc that likely gives T. weissflogii a survival advantage.[23]
Transport of cadmium
Cadmium is still considered lethal to phytoplankton in high amounts. Studies have shown that T. weissflogii has an initial toxic response to cadmium when exposed to it. The toxicity of the metal is reduced by the transcription and translation of phytochelatin, which are proteins that can bind and transport cadmium. Once bound by phytochelatin, cadmium is no longer toxic, and it can be safely transported to the CDCA enzyme.[22] It's also been shown that the uptake of cadmium via phytochelatin leads to a significant increase in CDCA expression.[22]
CDCA-like proteins
Other phytoplankton from different water sources have been tested for the presence of CDCA. It was found that many of them contain proteins that are homologous to the CDCA found in T. weissflogii.[22] This includes species from Great Bay, New Jersey as well as in the Pacific Ocean near the equator. In all species tested, CDCA-like proteins showed high levels of expression even in high concentrations of zinc and in the absence of cadmium.[22] The similarity between these proteins and the CDCA expressed by T. weissflogii varied, but they were always at least 67% similar.[22]
Carbon capture and sequestration
Carbonic anhydrase could in principle prove relevant to carbon capture. Some carbonic anhydrases can withstand temperatures up to 107 °C and extreme alkalinity (pH > 10).[27] A pilot run with the more stable CA on a flue stream that consisted of 12–13% mol composition CO₂ had a capture rate of 63.6% over a 60-hour period with no noticeable effects in enzyme performance. CA was placed in a N-methyldiethanolamine (MDEA) solution where it served to increase the concentration difference (driving force) of CO2 between the flue stream of the power plant and liquid phase in a liquid-gas contactor.[27]
See also
References
- Badger MR, Price GD (1994). "The role of carbonic anhydrase in photosynthesis". Annu. Rev. Plant Physiol. Plant Mol. Biol. 45: 369–392. doi:10.1146/annurev.pp.45.060194.002101.
- "PDB101: Molecule of the Month: Carbonic Anhydrase". RCSB: PDB-101. Retrieved 3 December 2018.
- Supuran CT (27 May 2004). Carbonic Anhydrases: Catalytic and Inhibition Mechanisms, Distribution and Physiological Roles. Taylor & Francis. doi:10.1201/9780203475300-5 (inactive 5 June 2020). ISBN 9780203475300.
- "Bohr Effect". www.pathwaymedicine.org. Retrieved 23 November 2019.
- "Le Chatelier's Principle". www.chemguide.co.uk. Retrieved 23 November 2019.
- "Britannica Dictionary".
- Maren TH (October 1967). "Carbonic anhydrase: chemistry, physiology, and inhibition". Physiological Reviews. 47 (4): 595–781. doi:10.1152/physrev.1967.47.4.595. PMID 4964060. S2CID 19954840.
- Biological Inorganic Chemistry. Structure and Reactivity. pp. section IX.1.3.1. p. 180.
- Lindskog S (1997). "Structure and mechanism of carbonic anhydrase". Pharmacology & Therapeutics. 74 (1): 1–20. doi:10.1016/S0163-7258(96)00198-2. PMID 9336012.
- Thatcher BJ, Doherty AE, Orvisky E, Martin BM, Henkin RI (September 1998). "Gustin from human parotid saliva is carbonic anhydrase VI". Biochemical and Biophysical Research Communications. 250 (3): 635–41. doi:10.1006/bbrc.1998.9356. PMID 9784398.
- Parkin G (February 2004). "Synthetic analogues relevant to the structure and function of zinc enzymes". Chemical Reviews. 104 (2): 699–767. doi:10.1021/cr0206263. PMID 14871139. S2CID 9857226.
- Breton S (July 2001). "The cellular physiology of carbonic anhydrases". JOP. 2 (4 Suppl): 159–64. PMID 11875253.
- Lovejoy DA, Hewett-Emmett D, Porter CA, Cepoi D, Sheffield A, Vale WW, Tashian RE (December 1998). "Evolutionarily conserved, "acatalytic" carbonic anhydrase-related protein XI contains a sequence motif present in the neuropeptide sauvagine: the human CA-RP XI gene (CA11) is embedded between the secretor gene cluster and the DBP gene at 19q13.3". Genomics. 54 (3): 484–93. doi:10.1006/geno.1998.5585. PMID 9878252.
- Boriack-Sjodin PA, Zeitlin S, Chen HH, Crenshaw L, Gross S, Dantanarayana A, et al. (December 1998). "Structural analysis of inhibitor binding to human carbonic anhydrase II". Protein Science. 7 (12): 2483–9. doi:10.1002/pro.5560071201. PMC 2143894. PMID 9865942.
- Unless else specified: Boron WF (2005). Medical Physiology: A Cellular And Molecular Approach. Elsevier/Saunders. ISBN 978-1-4160-2328-9. Page 638
- Hilvo M, Baranauskiene L, Salzano AM, Scaloni A, Matulis D, Innocenti A, et al. (October 2008). "Biochemical characterization of CA IX, one of the most active carbonic anhydrase isozymes". The Journal of Biological Chemistry. 283 (41): 27799–809. doi:10.1074/jbc.M800938200. PMID 18703501.
- Lehtonen J, Shen B, Vihinen M, Casini A, Scozzafava A, Supuran CT, et al. (January 2004). "Characterization of CA XIII, a novel member of the carbonic anhydrase isozyme family". The Journal of Biological Chemistry. 279 (4): 2719–27. doi:10.1074/jbc.M308984200. PMID 14600151.
- Hilvo M, Tolvanen M, Clark A, Shen B, Shah GN, Waheed A, et al. (November 2005). "Characterization of CA XV, a new GPI-anchored form of carbonic anhydrase". The Biochemical Journal. 392 (Pt 1): 83–92. doi:10.1042/BJ20051102. PMC 1317667. PMID 16083424.
- Sawaya MR, Cannon GC, Heinhorst S, Tanaka S, Williams EB, Yeates TO, Kerfeld CA (March 2006). "The structure of beta-carbonic anhydrase from the carboxysomal shell reveals a distinct subclass with one active site for the price of two". The Journal of Biological Chemistry. 281 (11): 7546–55. doi:10.1074/jbc.M510464200. PMID 16407248.
- So AK, Espie GS, Williams EB, Shively JM, Heinhorst S, Cannon GC (February 2004). "A novel evolutionary lineage of carbonic anhydrase (epsilon class) is a component of the carboxysome shell". Journal of Bacteriology. 186 (3): 623–30. doi:10.1128/JB.186.3.623-630.2004. PMC 321498. PMID 14729686.
- Del Prete S, Vullo D, Fisher GM, Andrews KT, Poulsen SA, Capasso C, Supuran CT (September 2014). "Discovery of a new family of carbonic anhydrases in the malaria pathogen Plasmodium falciparum—the η-carbonic anhydrases". Bioorganic & Medicinal Chemistry Letters. 24 (18): 4389–4396. doi:10.1016/j.bmcl.2014.08.015. hdl:10072/63103. PMID 25168745.
- Park H, McGinn PJ, More FM (19 May 2008). "Expression of cadmium carbonic anhydrase of diatoms in seawater". Aquatic Microbial Ecology. 51: 183–193. doi:10.3354/ame01192.
- Lane TW, Saito MA, George GN, Pickering IJ, Prince RC, Morel FM (May 2005). "Biochemistry: a cadmium enzyme from a marine diatom". Nature. 435 (7038): 42. Bibcode:2005Natur.435...42L. doi:10.1038/435042a. PMID 15875011.
- Bertini I, Gray H, Stiefel E, Valentine J (2007). Biological Inorganic Chemistry: Structure and Reactivity (First ed.). Sausalito, California: University Science Books. ISBN 978-1-891389-43-6.
- Sigel A, Sigel H, Sigel RK (2013). Cadmium from toxicity to essentiality. Dordrecht: Springer. ISBN 978-94-007-5179-8.
- Xu Y, Feng L, Jeffrey PD, Shi Y, Morel FM (March 2008). "Structure and metal exchange in the cadmium carbonic anhydrase of marine diatoms". Nature. 452 (7183): 56–61. Bibcode:2008Natur.452...56X. doi:10.1038/nature06636. PMID 18322527.
- Alvizo O, Nguyen LJ, Savile CK, Bresson JA, Lakhapatri SL, Solis EO, et al. (November 2014). "Directed evolution of an ultrastable carbonic anhydrase for highly efficient carbon capture from flue gas". Proceedings of the National Academy of Sciences of the United States of America. 111 (46): 16436–41. Bibcode:2014PNAS..11116436A. doi:10.1073/pnas.1411461111. PMC 4246266. PMID 25368146.
Further reading
- Lyall V, Alam RI, Phan DQ, Ereso GL, Phan TH, Malik SA, et al. (September 2001). "Decrease in rat taste receptor cell intracellular pH is the proximate stimulus in sour taste transduction". American Journal of Physiology. Cell Physiology. 281 (3): C1005-13. doi:10.1152/ajpcell.2001.281.3.C1005. PMID 11502578.