Bryophyte
Bryophytes are an informal group consisting of three divisions of non-vascular land plants (embryophytes): the liverworts, hornworts and mosses.[1] They are characteristically limited in size and prefer moist habitats although they can survive in drier environments.[2] The bryophytes consist of about 20,000 plant species.[3][4] Bryophytes produce enclosed reproductive structures (gametangia and sporangia), but they do not produce flowers or seeds. They reproduce via spores.[5] Bryophytes are usually considered to be a paraphyletic group and not a monophyletic group, although some studies have produced contrary results. Regardless of their status, the name is convenient and remains in use as an informal collective term. The term "bryophyte" comes from Greek βρύον, bryon "tree-moss, oyster-green" and φυτόν, phyton "plant".

Terminology
The term "Bryophyta" was first suggested by Braun (1964). G.M. Smith placed this group between Algae and Pteridophyta.[6]
Features
The defining features of bryophytes are:
- Their life cycles are dominated by the gametophyte stage
- Their sporophytes are unbranched
- They do not have a true vascular tissue containing lignin (although some have specialized tissues for the transport of water)[7]
Habitat
Bryophytes exist in a wide variety of habitats. They can be found growing in a range of temperatures (cold arctics and in hot deserts), elevations (sea-level to alpine), and moisture (dry deserts to wet rainforests).[8]
Bryophytes can grow where vascularized plants cannot because they do not depend on roots for an uptake of nutrients from soil. Bryophytes can survive on rocks and bare soil.[8]
Life cycle
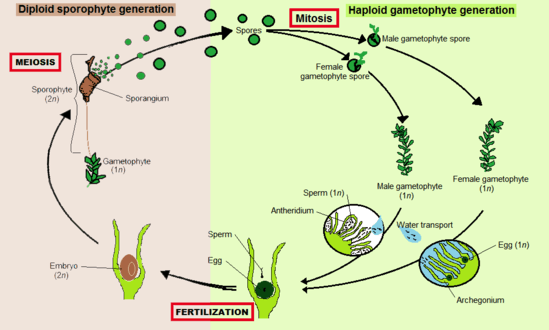
Like all land plants (embryophytes), bryophytes have life cycles with alternation of generations.[9] In each cycle, a haploid gametophyte, each of whose cells contains a fixed number of unpaired chromosomes, alternates with a diploid sporophyte, whose cell contain two sets of paired chromosomes. Gametophytes produce haploid sperm and eggs which fuse to form diploid zygotes that grow into sporophytes. Sporophytes produce haploid spores by meiosis, that grow into gametophytes.
Bryophytes are gametophyte dominant,[10] meaning that the more prominent, longer-lived plant is the haploid gametophyte. The diploid sporophytes appear only occasionally and remain attached to and nutritionally dependent on the gametophyte.[11] In bryophytes, the sporophytes are always unbranched and produce a single sporangium (spore producing capsule), but each gametophyte can give rise to several sporophytes at once.
The sporophyte develops differently in the three groups. Both mosses and hornworts have a meristem zone where cell division occur. In hornworts, the meristem starts at the base where the foot ends, and the division of cells is pushing the sporophyte body upwards. In mosses, the meristem is located between the capsule and the top of the stalk (seta), and produce cells downward, elongating the stalk and elevates the capsule. In liverworts the meristem is absent and the elongation of the sporophyte is caused almost exclusively by cell expansion.[12]
Liverworts, mosses and hornworts spend most of their lives as gametophytes. Gametangia (gamete-producing organs), archegonia and antheridia, are produced on the gametophytes, sometimes at the tips of shoots, in the axils of leaves or hidden under thalli. Some bryophytes, such as the liverwort Marchantia, create elaborate structures to bear the gametangia that are called gametangiophores. Sperm are flagellated and must swim from the antheridia that produce them to archegonia which may be on a different plant. Arthropods can assist in transfer of sperm.[13]
Fertilized eggs become zygotes, which develop into sporophyte embryos inside the archegonia. Mature sporophytes remain attached to the gametophyte. They consist of a stalk called a seta and a single sporangium or capsule. Inside the sporangium, haploid spores are produced by meiosis. These are dispersed, most commonly by wind, and if they land in a suitable environment can develop into a new gametophyte. Thus bryophytes disperse by a combination of swimming sperm and spores, in a manner similar to lycophytes, ferns and other cryptogams.
Sexuality
The arrangement of antheridia and archegonia on an individual bryophyte plant is usually constant within a species, although in some species it may depend on environmental conditions. The main division is between species in which the antheridia and archegonia occur on the same plant and those in which they occur on different plants. The term monoicous may be used where antheridia and archegonia occur on the same gametophyte and the term dioicous where they occur on different gametophytes.[14]
In seed plants, "monoecious" is used where flowers with anthers (microsporangia) and flowers with ovules (megasporangia) occur on the same sporophyte and "dioecious" where they occur on different sporophytes. These terms occasionally may be used instead of "monoicous" and "dioicous" to describe bryophyte gametophytes. "Monoecious" and "monoicous" are both derived from the Greek for "one house", "dioecious" and "dioicous" from the Greek for two houses. The use of the "oicy" terminology refers to the gametophyte sexuality of bryophytes as distinct from the sporophyte sexuality of seed plants.[14]
Monoicous plants are necessarily hermaphroditic, meaning that the same plant has both sexes.[14] The exact arrangement of the antheridia and archegonia in monoicous plants varies. They may be borne on different shoots (autoicous or autoecious), on the same shoot but not together in a common structure (paroicous or paroecious), or together in a common "inflorescence" (synoicous or synoecious).[14][15] Dioicous plants are unisexual, meaning that the same plant has only one sex.[14] All four patterns (autoicous, paroicous, synoicous and dioicous) occur in species of the moss genus Bryum.[15]
Classification and phylogeny
.jpg)

Traditionally, all living land plants without vascular tissues were classified in a single taxonomic group, often a division (or phylum). More recently, phylogenetic research has questioned whether the bryophytes form a monophyletic group and thus whether they should form a single taxon. Although a 2005 study supported the traditional view that the bryophytes form a monophyletic group,[16] by 2010 a broad consensus had emerged among systematists that bryophytes as a whole are not a natural group (i.e., are paraphyletic), although each of the three extant (living) groups is monophyletic.[17][18][19]
The three bryophyte clades (which may be treated as divisions) are the Marchantiophyta (liverworts), Bryophyta (mosses) and Anthocerotophyta (hornworts).[20] The vascular plants or tracheophytes form a fourth, unranked clade of land plants called the "Polysporangiophyta". In this analysis, hornworts are sister to vascular plants and liverworts are sister to all other land plants, including the hornworts and mosses.[19][21] Phylogenetic studies continue to produce conflicting results. In particular those based on gene sequences suggest the bryophytes are paraphyletic, whereas those based on the amino acid translations of the same genes suggest they are monophyletic. A 2014 study concluded that composition biases were responsible for these differences and that the bryophytes are monophyletic.[22] The issue remains unresolved.
Paraphyletic view
|
bryophytes |
_showing_clonal_plantlets_in_gemma_cups.jpg)
When extinct plants are taken into account, the picture is slightly altered. Some extinct land plants, such as the horneophytes, are not bryophytes, but also are not vascular plants because, like bryophytes, they do not have true vascular tissue. A different distinction is needed. In bryophytes, the sporophyte is a simple unbranched structure with a single spore-forming organ (sporangium). In all other land plants, the polysporangiophytes, the sporophyte is branched and carries many sporangia.[23][24] It has been argued that this contrast between bryophytes and other land plants is less misleading than the traditional one of non-vascular versus vascular plant, since many mosses have well-developed water-conducting vessels.[25] The contrast is shown in a slightly different cladogram:[26]
| land plants |
| |||||||||||||||||||||||||||
The term "bryophyte" thus refers to a grade of lineages defined primarily by what they lack. Compared to other living land plants, they lack vascular tissue containing lignin and branched sporophytes bearing multiple sporangia. The prominence of the gametophyte in the life cycle is also a shared feature of the three bryophyte lineages (extant vascular plants are all sporophyte dominant).
Other views
An alternative phylogeny, based on amino acids rather than genes, shows bryophytes as a monophyletic group:[22]
| embryophytes |
| ||||||||||||||||||
If this phylogeny proves correct, then the complex sporophyte of living vascular plants might have evolved independently of the simpler unbranched sporophyte present in bryophytes.[22] Other studies suggest a monophyletic group comprising liverworts and mosses, with hornworts being sister to vascular plants.[27]
Evolution
There have probably been several different terrestrialization events, in which originally aquatic organisms colonized the land, just within the lineage of the Viridiplantae.[28] Between 510 - 630 million years ago, however, land plants evolved from aquatic plants, specifically green algae. Molecular phylogenetic studies conclude that bryophytes are the earliest diverging lineages of the extant land plants.[29][1][30][31] They provide insights into the migration of plants from aquatic environments to land. A number of physical features link bryophytes to both land plants and aquatic plants.
Similarities to algae and vascular plants
Green algae, bryophytes and vascular plants all have chlorophyll a and b, and the chloroplast structures are similar.[32] Like algae and land plants, bryophytes also produce starch stored in the plastids and contain cellulose in their walls.[32]Distinct adaptations observed in bryophytes have allowed plants to colonize Earth's terrestrial environments. To prevent desiccation of plant tissues in a terrestrial environment, a waxy cuticle covering the soft tissue of the plant may be present, providing protection. In hornworts and mosses, stomata provide gas exchange between the atmosphere and an internal intercellular space system. The development of gametangia provided further protection specifically for gametes, the zygote and the developing sporophyte.[33] The bryophytes and vascular plants also have embryonic development Embryophytes which is not seen in green algae.[32] While bryophytes have no truly vascularized tissue, they do have organs that are specialized for specific functions, analogous for example to the functions of leaves and stems in vascular land plants.[32]
Bryophytes depend on water for reproduction and survival. In common with ferns, lycophytes a thin layer of water is required on the surface of the plant to enable the movement of the flagellated sperm between gametophytes and the fertilization of an egg.[33]
Comparative morphology
Summary of the morphological characteristics of the gametophytes of the three groups of bryophytes:
| Liverworts | Mosses | Hornworts | |
|---|---|---|---|
| Structure | Thalloid or foliose | Foliose | Thalloid |
| Symmetry | Dorsiventral or radial | Radial | Dorsiventral |
| Rhizoids | Unicellular | Pluricellular | Unicellular |
| Chloroplasts/cell | Many | Many | One |
| Protonemata | Reduced | Present | Absent |
| Gametangia (antheridia and archegonia) |
Superficial | Superficial | Immersed |
Summary of the morphological characteristics of the sporophytes of the three groups of bryophytes:
| Liverworts | Mosses | Hornworts | |
|---|---|---|---|
| Stomata | Absent | Present | Present |
| Structure | Small, without chlorophyll | Large, with chlorophyll | Large, with chlorophyll |
| Persistence | Ephemeral | Persistent | Persistent |
| Growth | Defined | Defined | Continuous |
| Seta | Present | Present | Absent |
| Capsule form | Simple | Differentiated (operculum, peristome) |
Elongated |
| Maturation of spores | Simultaneous | Simultaneous | Graduate |
| Dispersion of spores | Elaters | Peristome teeth | Pseudo-elaters |
| Columella | Absent | Present | Present |
| Dehiscence | Longitudinal or irregular | Transverse | Longitudinal |
Uses
Environmental
- Soil Conditioning
- Bioindicators
- Moss gardens
- Pesticides
Characteristics of bryophytes make them useful to the environment. Depending on the specific plant texture, bryophytes have been shown to help improve the water retention and air space within soil.[34] Bryophytes are used in pollution studies to indicate soil pollution (such as the presence of heavy metals), air pollution, and UV-B radiation.[34] Gardens in Japan are designed with moss to create peaceful sanctuaries.[34] Some bryophytes have been found to produce natural pesticides. The liverwort, Plagiochila, produces a chemical that is poisonous to mice.[34] Other bryophytes produce chemicals that are antifeedants which protect them from being eaten by slugs.[34] When Phythium sphagnum is sprinkled on the soil of germinating seeds, it inhibits growth of "damping off fungus" which would otherwise kill young seedlings.[35]

Commercial
- Fuel
- Packaging
- Wound Dressing
Peat is a fuel produced from dried bryophytes, typically Sphagnum. Bryophytes' antibiotic properties and ability to retain water make them a useful packaging material for vegetables, flowers, and bulbs.[34] Also, because of its antibiotic properties, Sphagnum was used as a surgical dressing in World War I.[34]
See also
- Anthocerotophyta (hornworts)
- Bryophyta (mosses)
- Embryophyte
- Marchantiophyta (liverworts)
- Plant sexuality
- List of British county and local bryophyte floras
References
- Hedges, S. Blair (November 2002). "The origin and evolution of model organisms". Nature Reviews Genetics. 3 (11): 838–849. doi:10.1038/nrg929. PMID 12415314.
- Levetin, Estelle; McMahon, Karen (2012). Plants and Society. New York, NY: McGraw-Hill. p. 139. ISBN 978-0-07-352422-1.
- "Bryophytes (Mosses and liverworts) — The Plant List". www.theplantlist.org. Retrieved 2017-04-11.
- "What are Bryophytes". Southern Illinois University Carbondale.
- Vanderpoorten, Alain; Goffinet, Bernard (2009). Introduction to Bryophytes. Cambridge: Cambridge University Press. p. 3. ISBN 978-0-511-54013-4.
- Smith, G.M. (1955). Cryptogamic Botany. 2 (2nd ed.). New York: McGraw-Hill.
- Lucas, William J.; Groover, Andrew; Lichtenberger, Raffael; Furuta, Kaori; Yadav, Shri-Ram; Helariutta, Ykä; He, Xin-Qiang; Fukuda, Hiroo; Kang, Julie; Brady, Siobhan M.; Patrick, John W. (April 2013). "The Plant Vascular System: Evolution, Development and Functions F". Journal of Integrative Plant Biology. 55 (4): 294–388. doi:10.1111/jipb.12041. hdl:10261/76903. PMID 23462277.
- "Habitats - ecology - bryophyte". www.anbg.gov.au. Retrieved 2017-04-12.
- Ligrone, Roberto; Duckett, Jeffrey G.; Renzaglia, Karen S. (April 2012). "Major transitions in the evolution of early land plants: a bryological perspective". Annals of Botany. 109 (5): 851–871. doi:10.1093/aob/mcs017. PMC 3310499. PMID 22356739.
- CM Sean Carrington (2013-11-04). "Bryophytes". Retrieved 2020-03-05.
- Johnson, M G; Shaw, A J (24 February 2016). "The effects of quantitative fecundity in the haploid stage on reproductive success and diploid fitness in the aquatic peat moss Sphagnum macrophyllum". Heredity. 116 (6): 523–530. doi:10.1038/hdy.2016.13. PMC 4868265. PMID 26905464.
- Plant Development and Evolution
- Cronberg, N.; Natcheva, R.; Hedlund, K. (2006). "Microarthropods Mediate Sperm Transfer in Mosses". Science. 313 (5791): 1255. doi:10.1126/science.1128707. PMID 16946062. S2CID 11555211.
- Glime, J.M. & Bisang, I. (2014). "Sexuality: Its Determination (Ch. 3-1)" (PDF). In Glime, J.M. (ed.). Bryophyte Ecology. Volume 1 Physiological Ecology. Michigan Technological University and the International Association of Bryologists. Retrieved 2014-11-09.
- Watson, E.V. (1981). British Mosses and Liverworts (3rd ed.). Cambridge University Press. p. 7. (Watson uses the "oecy" terms rather than the "oicy" terms.)
- Goremykin, V. V. & Hellwig, F. H. (2005). "Evidence for the most basal split in land plants dividing bryophyte and tracheophyte lineages". Plant Systematics and Evolution. 254 (1–2): 93–103. doi:10.1007/s00606-005-0337-1.
- Konrat, M.; Shaw, A.J.; Renzaglia, K.S. (2010). "A special issue of Phytotaxa dedicated to Bryophytes: The closest living relatives of early land plants". Phytotaxa. 9: 5–10. doi:10.11646/phytotaxa.9.1.3.
- Troitsky, A. V.; Ignatov, M. S.; Bobrova, V. K.; Milyutina, I. A. (December 2007). "Contribution of genosystematics to current concepts of phylogeny and classification of bryophytes". Biochemistry (Moscow). 72 (12): 1368–1376. doi:10.1134/s0006297907120115. PMID 18205621.
- Knoop, Volker (31 December 2010). "Looking for sense in the nonsense: a short review of non-coding organellar DNA elucidatingn the phylogeny of bryophytes". Bryophyte Diversity and Evolution. 31 (1): 51–60. doi:10.11646/bde.31.1.10.
- "GLOSSARY B". Archived from the original on 2009-04-02. Retrieved 2009-03-26.
- Qiu, Yin-Long; Li, Libo; Wang, Bin; Chen, Zhiduan; Knoop, Volker; Groth-Malonek, Milena; Dombrovska, Olena; Lee, Jungho; Kent, Livija; Rest, Joshua; Estabrook, George F.; Hendry, Tory A.; Taylor, David W.; Testa, Christopher M.; Ambros, Mathew; Crandall-Stotler, Barbara; Duff, R. Joel; Stech, Michael; Frey, Wolfgang; Quandt, Dietmar; Davis, Charles C. (17 October 2006). "The deepest divergences in land plants inferred from phylogenomic evidence". Proceedings of the National Academy of Sciences of the United States of America. 103 (42): 15511–15516. Bibcode:2006PNAS..10315511Q. doi:10.1073/pnas.0603335103. PMC 1622854. PMID 17030812.
- Cox, Cymon J.; Li, Blaise; Foster, Peter G.; Embley, T. Martin; Civáň, Peter (2014). "Conflicting Phylogenies for Early Land Plants are Caused by Composition Biases among Synonymous Substitutions". Systematic Biology. 63 (2): 272–279. doi:10.1093/sysbio/syt109. PMC 3926305. PMID 24399481.
- Kenrick, Paul & Crane, Peter R. (1997a). The Origin and Early Diversification of Land Plants: A Cladistic Study. Washington, D.C.: Smithsonian Institution Press. ISBN 978-1-56098-730-7.
- Kenrick, P. & Crane, P.R. (1997b). "The origin and early evolution of plants on land". Nature. 389 (6646): 33–39. Bibcode:1997Natur.389...33K. doi:10.1038/37918.
- Bell, N. E. & Hyvönen, J. (2010). "Phylogeny of the moss class Polytrichopsida (BRYOPHYTA): Generic-level structure and incongruent gene trees". Molecular Phylogenetics and Evolution. 55 (2): 381–398. doi:10.1016/j.ympev.2010.02.004. PMID 20152915.
- Crane, Peter R.; Herendeen, Patrick; Friis, Else Marie (October 2004). "Fossils and plant phylogeny". American Journal of Botany. 91 (10): 1683–1699. doi:10.3732/ajb.91.10.1683. PMID 21652317.
- Karol, Kenneth G.; Arumuganathan, Kathiravetpillai; Boore, Jeffrey L.; Duffy, Aaron M.; Everett, Karin DE; Hall, John D.; Hansen, S.K.; Kuehl, Jennifer V.; Mandoli, Dina F.; Mishler, Brent D.; Olmstead, Richard G.; Renzaglia, Karen S. & Wolf, Paul G. (2010). "Complete plastome sequences of Equisetum arvense and Isoetes flaccida: implications for phylogeny and plastid genome evolution of early land plant lineages". BMC Evolutionary Biology. 10 (1): 321. doi:10.1186/1471-2148-10-321. PMC 3087542. PMID 20969798.
- Gerrienne, Philippe; Servais, Thomas; Vecoli, Marco (April 2016). "Plant evolution and terrestrialization during Palaeozoic times—The phylogenetic context". Review of Palaeobotany and Palynology. 227: 4–18. doi:10.1016/j.revpalbo.2016.01.004.
- Konrat, M.; Shaw, A.J.; Renzaglia, K.S. (2010). "A special issue of Phytotaxa dedicated to Bryophytes: The closest living relatives of early land plants". Phytotaxa. 9: 5–10. doi:10.11646/phytotaxa.9.1.3.
- Karol, Kenneth G.; Arumuganathan, Kathiravetpillai; Boore, Jeffrey L.; Duffy, Aaron M.; Everett, Karin DE; Hall, John D.; Hansen, S.K.; Kuehl, Jennifer V.; Mandoli, Dina F.; Mishler, Brent D.; Olmstead, Richard G.; Renzaglia, Karen S. & Wolf, Paul G. (2010). "Complete plastome sequences of Equisetum arvense and Isoetes flaccida: implications for phylogeny and plastid genome evolution of early land plant lineages". BMC Evolutionary Biology. 10 (1): 321. doi:10.1186/1471-2148-10-321. PMC 3087542. PMID 20969798.
- Shaw, A. Jonathan; Szövényi, Péter; Shaw, Blanka (March 2011). "Bryophyte diversity and evolution: Windows into the early evolution of land plants". American Journal of Botany. 98 (3): 352–369. doi:10.3732/ajb.1000316. PMID 21613131.
- Everet, Ray; Eichhorn, Susan (2013). Biology of Plants. W.H.Freeman and Company Publishers.
- Purcell, Adam. "Bryophytes". Basic Biology.
- Glime, Janice. "Economic and Ethnic Uses of Bryophytes" (PDF). harvard.edu.
- Wolffhechel, H. (April 1988). "The suppressiveness of sphagnum peat to Pythium spp". Acta Horticulturae (221): 217–222. doi:10.17660/actahortic.1988.221.22.
Bibliography
- Lesica, P.; McCune, B.; Cooper, S. V.; Hong, W. S. (1991). "Differences in lichen and bryophyte communities between old-growth and managed second-growth forests in the Swan Valley, Montana". Canadian Journal of Botany. 69 (8): 1745–1755. doi:10.1139/b91-222.
External links
| Wikimedia Commons has media related to Bryophytes. |
| Look up bryophyte in Wiktionary, the free dictionary. |
| Wikisource has the text of the 1911 Encyclopædia Britannica article Bryophyta. |
- Andrew's Moss Site Photos of bryophytes
- 27-May-2013 Centuries-old frozen plants revived, 400-year-old bryophyte specimens left behind by retreating glaciers in Canada are brought back to life in the laboratory.
- Farge, Catherine La; Williams, Krista H.; England, John H. (22 May 2013). "Regeneration of Little Ice Age bryophytes emerging from a polar glacier with implications of totipotency in extreme environments". Proceedings of the National Academy of Sciences. 110 (24): 9839–9844. Bibcode:2013PNAS..110.9839L. doi:10.1073/pnas.1304199110. PMC 3683725. PMID 23716658.
- Magill, R. E., ed. (1990). Glossarium polyglottum bryologiae. A multilingual glossary for bryology. Monographs in Systematic Botany from the Missouri Botanical Garden, v. 33, 297 pp. Online version: Internet Archive.