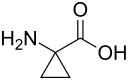
1-Aminocyclopropane-1-carboxylic acid
1-Aminocyclopropane-1-carboxylic acid (ACC) is a disubstituted cyclic α-amino acid in which a three-membered cyclopropane ring is fused to the Cα atom of the amino acid.
 | |
| Names | |
|---|---|
| Other names
1-Aminocyclopropanecarboxylic acid | |
| Identifiers | |
3D model (JSmol) |
|
| Abbreviations | ACC |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| ECHA InfoCard | 100.108.227 |
| KEGG | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| Properties | |
| C4H7NO2 | |
| Molar mass | 101.1 c |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
ACC plays an important role in the biosynthesis of the plant hormone Ethylene as a plant hormone#ethylene.[2][3] It is synthesized by the enzyme ACC synthase ( EC 4.4.1.14) from methionine and converted to ethylene by ACC oxidase (EC 1.14.17.4).[4] ACC can be used by soil microorganisms (both bacteria and fungi) as a source of nitrogen and carbon.[5] As such, using ACC to incubate soils has been proven to induce the gene abundance encoding ACC-deaminases, which may have positive consequences on plant growth and stress tolerance.[6]
ACC is also an exogenous partial agonist of the mammalian NMDA receptor.[7]
In 2019, the United States Environmental Protection Agency issued notice of an application for an Experimental use permit to be issued for use of ACC as a pesticide.[8]
References
- Caspi R, Foerster H, Fulcher CA, Hopkinson R, Ingraham J, Kaipa P, Krummenacker M, Paley S, Pick J, Rhee SY, Tissier C, Zhang P, Karp PD (2006). "MetaCyc: a multiorganism database of metabolic pathways and enzymes". Nucleic Acids Res. 34 (Database issue): D511–6. doi:10.1093/nar/gkj128. PMC 1347490. PMID 16381923.
- Yang S, Hoffman N (1984). "Ethylene biosynthesis and its regulation in higher plants". Annu. Rev. Plant Physiol. 35: 155–189. doi:10.1146/annurev.pp.35.060184.001103.
- Kende H (1993). "Ethylene biosynthesis". Annu. Rev. Plant Physiol. 44: 283–307. doi:10.1146/annurev.pp.44.060193.001435.
- Kende H (1989). "Enzymes of Ethylene Biosynthesis". Plant Physiol. 91 (1): 1–4. doi:10.1104/pp.91.1.1. PMC 1061940. PMID 16666977.
- Schenk, Peer M.; Singh, Brajesh; Dennis, Paul G.; Crawford, Mark; Yan, Lijuan; Delgado-Baquerizo, Manuel; Carvalhais, Lilia C.; Khan, Muhammad Yahya; Liu, Hongwei (2019-05-03). "Soil amendments with ethylene precursor alleviate negative impacts of salinity on soil microbial properties and productivity". Scientific Reports. 9 (1): 6892. doi:10.1038/s41598-019-43305-4. ISSN 2045-2322. PMC 6499801. PMID 31053834.
- Schenk, Peer M.; Singh, Brajesh; Dennis, Paul G.; Crawford, Mark; Yan, Lijuan; Delgado-Baquerizo, Manuel; Carvalhais, Lilia C.; Khan, Muhammad Yahya; Liu, Hongwei (2019-05-03). "Soil amendments with ethylene precursor alleviate negative impacts of salinity on soil microbial properties and productivity". Scientific Reports. 9 (1): 6892. doi:10.1038/s41598-019-43305-4. ISSN 2045-2322. PMC 6499801. PMID 31053834.
- Inanobe A, Furukawa H, Gouaux E (2005). "Mechanism of partial agonist action at the NR1 subunit of NMDA receptors". Neuron. 47 (1): 71–84. doi:10.1016/j.neuron.2005.05.022. PMID 15996549.
- "Pesticide Experimental Use Permit; Receipt of Application; Comment Request" (PDF). Federal Register. 84 (152): 38624. August 7, 2019 – via www.govinfo.gov.