UV filter


UV filters are individual compounds or mixtures that block or absorb ultraviolet (UV) light. UV filters are used in sunscreens to protect skin or in photography to reduce the level of ultraviolet light that strikes the recording medium. UV filters can undergo transformation into less protective or more toxic products. These transformation products can have health and environmental effects.
Background
Historically, photographic films were mostly sensitive to UV light, which caused haziness or fogginess, and in color films a bluish hue. Therefore, as a standard, a UV (blocking) filter was used, transparent to visible light while filtering out shorter ultraviolet wavelengths. However, newer photographic film and digital cameras are highly insensitive to UV wavelengths. UV filters are sometimes referred to as L37 or L39 filters, depending on the wavelengths of light that they filter out; an L37 filter removes ultraviolet light with a wavelength shorter than 370 nm, whereas an L39 filter eliminates light with a wavelength shorter than 390 nm.
Applications in printing and photography
UV filters span the color spectrum and are used for a wide variety of applications. Ortho Red and Deep Ortho Red lights are commonly used in diffusion transfer, typesetting films/paper, and other applications dealing with orthochromatic materials. Yellow Gold, Yellow, Lithostar Yellow, and Fuji Yellow filters or safelights provide safe workspaces for contact proofing applications like screen printing and platemaking. Pan Green, Infrared Green, and Dark Green filters or safelights are commonly used in scanning applications, work with panchromatic film ,and papers and x-rays.[1]
Many photographers and cinematographers still use UV filters as a protection for their lenses' glass and coating, due to their low cost and lack of effect on the exposure of the shot. However, UV filters (in particular filters lacking optical coating) may introduce lens flare and have an adverse impact on contrast and sharpness, especially when a strong light source is present.[2]
However, in photography, the term "UV filter" can be also misused as a filter that passes UV light while blocking other wavelengths in the light spectrum, in the same way the term "IR filter" is also sometimes misused. The correct names for such filters though, are "UV pass filter" and "IR pass filter". This is a very specialized area in photography.
Applications in personal care products
Excessive UV radiation can expose humans to various risks, including skin diseases such as sunburns, photo-aging, and skin cancers.[3] UV radiation can be classified as UVA (320-400 nm), UVB (290-320 nm) and UVC (200-280 nm ). Ultraviolet absorbing compounds are the active ingredients of sunscreen products and other personal care products (e.g. lipsticks, shampoos, hair sprays, body washes, toilet soaps, and insect repellents).[4]
Chemical filters are generally used in combination in order to get sufficient protection against UV.[4] They protect humans from the serious effects of UV radiation by absorbing, reflecting or scattering it.[5] The reflection and scattering effecst are done by physical UV filters which are inorganic UV filters such as titaniumdioxide (TiO2) and zinc oxide (ZnO). Absorption is accomplished by organic UV filters which are known as chemical UV filters (mainly UVB).[6]
Examples of Organic UV filters[7]
| Benzophenones | Triazines | Cinnamates |
|---|---|---|
| Benzophenone-3 (BP3) | Ethylhexyltriazone (OT) | Ethylhexyl methoxycinnamate (OMC) |
| Benzophenone-4 (BP4) | Diethylhexyl butamido triazone (DBT) | Isoamyl p-methoxycinnamate (IMC) = amiloxate |
| Salicylates | Bis-ethylhexyloxyphenol methoxyphenyl triazine (EMT) | Camphor derivatives |
| Homosalate (HMS) | Benzotriazoles | Terephtalydene dicamphor sulfonic acid (PDSA) |
| 2-ethylhexyl salicylate (EHS) | Drometrizole trisiloxane (DRT) | 3-benzylidene camphor (3BC) |
| P-Aminobenzoic acid and derivatives | Methylene bis-benzotriazolyl tetramethylbutylpheno
(biscotrizole) (MBP) |
Benzylidene camphor sulfonic acid (BCSA) |
| Ethylhexyl dimethyl PABA (OD-PABA) | Dybenzoyl methane derivatives | 4-methylbenzylidene camphor (4-MBC) |
| 4-p-aminobenzoic acid (PABA) | Butyl methoxydibenzoyl methane (BM-DBM) | Polyacrylamidomethyl benzylidene camphor (PBC) |
| Benzimidazole derivatives | Camphor benzalkonium methosulfate (CBM) | |
| Phenylbenzimidazole sulfonic acid (PMDSA) | ||
| Disodium phenyl dibenzimidazole tetrasulfonate
(bisdisulizole disodium) |
Environmental aspects
UV filters are being used increasingly due to growing concern about UV radiation and skin cancer, especially as a result of ozone depletion.[5] The levels of certain UV-filters in sunscreens vary from 0.5 to 10%, sometimes reaching 25%.[8] They enter the environment directly through bathing activities in oceans, rivers and lakes or through industrial waste water discharge. They are indirectly introduced through domestic water discharge during showering, bathing or urine excretion or through waste water treatment.Waste water treatment plants (WWTP) are not very effective at removing these contaminants.[8] Several UV filters have been detected at ppb or ppt levels in surfacewater and wastewater, with maximum concentrations in summer time.[9][10] They are lipophilic and tend to accumulate in the aquatic environment’s soils and sediments as well as in the food chain. Several studies have actually shown the presence of UV filters in aquatic organisms. The 4-methyl-benzylidenecamphor was detected in the muscle tissue of trout in Swiss and German waters, while traces of ethylhexylmethoxy cinnamate and octocrylene were found in shellfish in the Mediterranean and Atlantic coasts of France.[11][12] Furthermore, eighteen organic sunscreens were found in sediments of Japanese rivers and lakes, at concentrations ranging from 2 to about 3000 ng/g.[13] The accumulation of organic UV filters in living organisms is of major concern because some of them (and their metabolites) can act as endocrine disruptors both in vitro and in vivo.[14] In addition, Goksøyr et al.(2009) reported concentrations of organic UV-filters in openwaters of the Pacific Ocean, providing evidence of the persistence and wide dispersion of these components in the marine environment.[15]
Their continuous release to the environment has prompted them to be considered as a new class of pollutants. UV-filters are not always stable under environmental conditions.Water in natural reservoirs is always subjected to sun irradiation, while swimming-pool water is required to be disinfected by chlorination, bromination, ozonation, or UV-irradiation.[16] UV filters can undergo degradation and transformation to more toxic products. For example, Avobenzone undergoes transformation in the presence of chlorinated disinfection products and UV radiation to substituted chlorinated phenols and acetophenones, which are known for their toxicity.[8]
Some organic UV filters under UV radiation can generate reactive Oxygen Species (ROS) (OH, H2O2) (e.g. BP-3, octocrylene (OCR), OMC, phenylbenzimidazole sulphonic acid (PBS), PABA, etc.).The levels of H2O2 in waters of Mediterranean beach reached 270 nM/day as a result of Sunscreens.[17] H2O2 as an oxidizing specie is responsible of damaging lipids, proteins and DNA and generating high stress levels in marine organisms.[18] Inorganic UV-filters (i.e. TiO2) can also generate ROS, which is toxic for the marine phytoplankton .The toxicity of nano-TiO2 is due to its photochemical properties under solar radiation that depend on the radiation intensity, the crystalline structure and the concentration of the nanoparticles. In addition, the inert coating layer of the nanoparticles which protects against photoreactivity is dissolved in aquatic environments after being released from sunscreens.[15]
Mechanisms of transformation
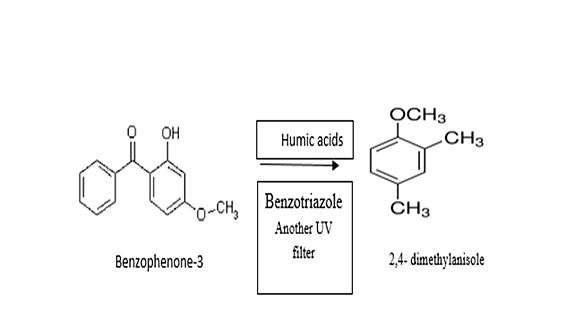
Photolysis
Photolysis is the main abiotic route for transformation of UV filters. Photolysis dissociates the organic filters into free radicals.[7]
Photolysis can be direct or indirect. The direct way occurs when the chromophore of the organic filters absorbs sunlight at certain wavelengths. The indirect pathway occurs in the presence of a photo-sensitizer. Dissolved organic matter (DOM) in the surface waters act as the photo-sensitizers and produce reactive photooxidants as hydroxyl radicals, peroxyl radicals and singlet oxygen.
The photolysis of sunscreen products is more complicated than the behavior of individual UV filters,as shown by this example. In the presence of other UV filter, Benzotriazole, and humic acids, Benzophenone -3 degradation was observed through the loss of hydroxyl and benzoyl functional groups resulting in the formation of 2,4 dimethyl anisole.[19]
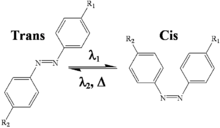
Photoisomerization
Photoisomerization can result in products that absorb less UV light than the parent compound.[20] This is evidenced by cinnamates, salicylates, benzylidine camphor and dibenzoylmethane derivatives. Octyl methoxycinnamate (OMC) can undergo photoisomerization, photodegradation and photodimerization to obtain several dimer and cyclodimers isomers.Most commercial products are trans isomers by they exist in the environment as a mixture of trans and cis isomers upon exposure to UV radiation due to the presence of the C=C double bond adjacent to the aromatic rings. The isomers may have identical physico-chemical properties, but they may differ in biological behavior and effects.[7]
Disinfection by-product
Swimming pool water is usually disinfected by chlorination, bromination, ozonation or UV radiation. Upon the presence of some UV filters such as Avobenzone in swimming pools, toxic products are produced as a result of the interaction between Avobenzone and the active chlorine and UV radiation.[8]
Fate of some Organic UV filters
Benzophenones

Benzophenones (BPs) are widely used in UV filters, fragrance enhancers,and plastic additives. The major sources of BP-3 are reported to be human recreational activities and wastewater treatment plant (WWTP) effluents.The anionic forms of both BP-3 and 4-OH-BP3 can undergo direct photodegradation. The photolytic rates of both compounds in natural waters are faster than those in pure water. Radical scavenging experiments revealed that triplet-excited dissolved organic matter (3DOM*) was responsible for the indirect photodegradation of BP-3 and 4-OH-BP3 in seawater, whereas in freshwater, the indirect photodegradation of these two compounds was attributed to Dissolved Organic Matter and OH radical.[21]
P-aminobenzoic acid (PABA)
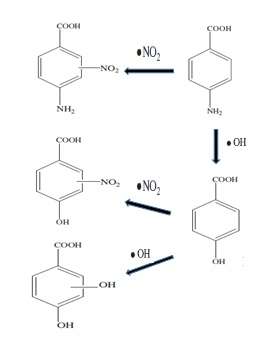
P-amino benzoic acid is one of the earliest UV filters to be used in sunscreens (1943). It was used in concentrations up to 5%. It was discovered by 1982 that PABA increases the formation of a particular DNA defect in human cells. The aquatic environment has been contaminated by PABA from sunscreens. The photochemical fate of PABA may be impacted by water constituents, e.g., NO3−, dissolved organic matter (DOM), and HCO3−.[22] PABA undergoes both direct and indirect photolysis in the solution with the presence of NO3. Direct photolysis accounts for 25% of the degradation of PABA and is considered a secondary pathway. On the other hand, indirect photolysis was the dominant pathway.
Zhou and Mopper showed that nitrate enhanced the photodegradation of PABA by factor of 2. However, in the presence of free radical scavengers such as carbonate forms and natural organic matter (NOM), the photodegradation of PABA decreased. It was proposed that the indirect photolysis of PABA was mainly due to the NO3 photolysis product •OH.
The Bicarbonate anion is abundant in water. Bicarbonate caused 10% of •OH scavenging. The reaction between bicarbonate and the •OH yields carbonate radical (•CO3), which is less reactive than •OH. In natural waters •CO3 can reach a higher steady-state concentration than •OH because of its lower reactivity. The enhancement of PABA photolysis by bicarbonate is due to carbonate radicals.[22]
Water-soluble NOM is composed of organic acids. These organic acids are mainly humic substances, which can be categorized into fulvic and humic acid fraction. NOM favors the indirect photolysis of PABA through absorbing the sunlight and weakening its intensity.
Two reactions can take place during the degradation of PABA in presence of nitrate in water as shown in the figure .Three of the four products contain phenolic groups and may thus be estrogenic. So the hazardous byproducts generating during the PABA photoreaction should be concerned for its estrogenicity.
4-tert-butyl-4’-methoxydibenzoylmethane (avobenzone)
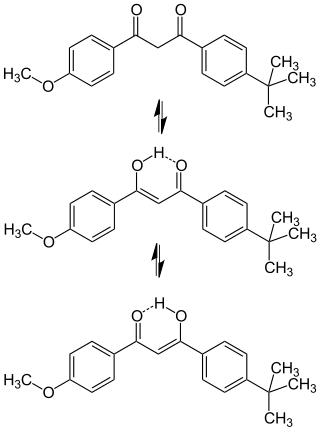
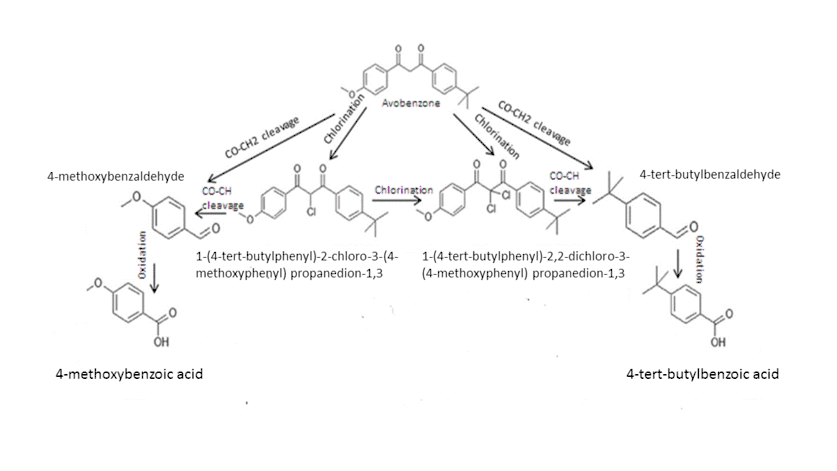
4-tert-butyl-4’-methoxydibenzoylmethane, known as avobenzone belong to dibenzoylmethanes. It is one of the most UVA (400-320 nm) filters that are used in sunscreens formulations.It is sold under the trade names Parsol 1789 or Eusolex 9020. Avobenzone exists in two tautomeric forms: enol and keto ones.In sunscreen formulations, avobenzone exists predominantly in the enol form, which has a maximum absorption at wavelengths ranging from 350 to 365 nm depending on the solvent used. The double bond of the enolic form was shown to be more reactive in conditions of aquatic chlorination, than the aromatic ring.In chlorinated aquatic environment, Avobenzone undergoes transformation to two corresponding aldehydes and acids, as shown in the figure. Both aldehydes are formed as a result of the CO-CH2 bond .They are less stable in the oxidative conditions and easily transform into the corresponding acids.
Chlorinated acetophenone derivatives are also formed due to the cleavage of the same CO-CH2 bond. Chlorinated acetophenone derivatives are tear gases, trigger dermatitis and some other health problems.It was reported that chlorination of the original Avobenzone into the aromatic ring position is less possible. The cleavage of CO-Ar bond results in formation of 4-chloroanisole.[8]
Ethylhexyl methoxycinnamate (EHMC)
Ethylhexyl methoxy cinnamate (EHMC) is one of the most famous UVB filters used worldwide.It is known as Eusolex 2292 and Uvinul MC80. It is included in the so-called High Production Volume Chemicals (HPVC) list, that includes chemicals produced or imported in the EU at a rate of more than 1000 tons per year.The life time of the EHMC was predicted to be from hours to few days. EHMC is well tolerated by the skin. However, it has some side effects including its ability to produce reactive oxygen species (ROS) and penetrate in the human skin after exposure to UV light .EHMC has also been found in shellfish, fish and cormorants at ng/g levels, which suggests that it can be accumulated in the food chain.[23] EHMC was proved to be responsible for coral bleaching by promoting viral infections.[24] From the toxicological point of view, EHMC has estrogenic properties both in vitro and in vivo . For instance, exposure to this compound caused the increase of the weight of the uterus in rats. Prenatal exposure to EHMC can affect both the reproductive and neurological development in the offspring of rats, which can be a cause for concern because humans are routinely exposed to this compound through the use of sunscreens and other cosmetics.
The main transformation pathway for EHMC is photolysis. Direct photolysis represents the dominant transformation pathway. on the other hand, the indirect photolysis due to •OH is negligible and due to dissolved organic matter will be a secondary route. Four transformation products were detected for EHMC upon exposure to UV radiation.4-methoxybenzaldehyde (MOBA)and 4-methoxycinnamic acid are two transformation products of EHMC via dealkylation.The intermediate MOBA is more toxic than EHMC towards the bacteria.
Environmental impact of some UV filters
Coral bleaching
_with_signs_of_bleaching_or_crown-of-thorns_starfish_damage.jpg)
According to the rough estimate of 78 million tourists per year in coral reef areas. An estimated amount of Sunscreens between 16,000 and 25,000 tons are used annually in the tropical countries. 25% of this amount is washed off during bathing activities, leading to release of 4,000-6,000 tons/year in the reef areas. This results in threatening of 10% of the world reefs by sunscreen induced coral bleaching.[24] Sunscreens can significantly enhance viral production in seawater.[24]
UV filters have shown severe effects on coral reefs by bleaching corals at very low concentrations. As a result, small quantities of sunscreens result in the production of large amounts of coral mucous within 18-48hr and bleaching of hard corals with in 96 hrs. Among the UV filters that result in coral bleaching according to studies are: ethylhexylmethoxycinnamate, benzophenone-3 and 4-methylbenzylidene camphor even in very low concentrations. Bleaching was favored by higher temperature which act as synergistic factor. Experiments showed that the coral bleaching was not dose dependent, so it can occur upon exposure to very small amount.[24]
See also
References
- ↑ "Fluorescent Safety Lamp Selection Chart - Encapsulite".
- ↑ Thom Hogan. "Filtration 101". bythom.com. Retrieved 13 October 2009.
- ↑ Pathak, Madhu A (1987). "Sunscreens and Their Use in the Preventive Treatment of Sunlight-Induced Skin Damage". The Journal of Dermatologic Surgery and Oncology. 13 (7): 739–50. doi:10.1111/j.1524-4725.1987.tb00544.x. PMID 3298346.
- 1 2 Kim, Sujin; Choi, Kyungho (2014). "Occurrences, toxicities, and ecological risks of benzophenone-3, a common component of organic sunscreen products: A mini-review". Environment International. 70: 143–57. doi:10.1016/j.envint.2014.05.015. PMID 24934855.
- 1 2 Díaz-Cruz, M. Silvia; Barceló, Damià (June 2009). "Chemical analysis and ecotoxicological effects of organic UV-absorbing compounds in aquatic ecosystems". TrAC Trends in Analytical Chemistry. Applying combinations of chemical analysis and biological effects to environmental and food samples - II. 28 (6): 708–17. doi:10.1016/j.trac.2009.03.010.
- ↑ Gasparro, Francis P; Mitchnick, Mark; Nash, J. Frank (1998). "A Review of Sunscreen Safety and Efficacy". Photochemistry and Photobiology. 68 (3): 243–56. doi:10.1562/0031-8655(1998)068<0243:arossa>2.3.co;2. PMID 9747581.
- 1 2 3 Silvia Díaz-Cruz, M.; Llorca, Marta; Barceló, Damià; Barceló, Damià (November 2008). "Organic UV filters and their photodegradates, metabolites and disinfection by-products in the aquatic environment". TrAC Trends in Analytical Chemistry. Advanced MS Analysis of Metabolites and Degradation Products - I. 27 (10): 873–87. doi:10.1016/j.trac.2008.08.012.
- 1 2 3 4 5 Trebše, Polonca; Polyakova, Olga V; Baranova, Maria; Kralj, Mojca Bavcon; Dolenc, Darko; Sarakha, Mohamed; Kutin, Alexander; Lebedev, Albert T (2016). "Transformation of avobenzone in conditions of aquatic chlorination and UV-irradiation". Water Research. 101: 95–102. doi:10.1016/j.watres.2016.05.067. PMID 27258620.
- ↑ Poiger, Thomas; Buser, Hans-Rudolf; Balmer, Marianne E; Bergqvist, Per-Anders; Müller, Markus D (2004). "Occurrence of UV filter compounds from sunscreens in surface waters: Regional mass balance in two Swiss lakes". Chemosphere. 55 (7): 951–63. doi:10.1016/j.chemosphere.2004.01.012. PMID 15051365.
- ↑ Magi, Emanuele; Scapolla, Carlo; Di Carro, Marina; Rivaro, Paola; Ngoc Nguyen, Kieu Thi (2013). "Emerging pollutants in aquatic environments: Monitoring of UV filters in urban wastewater treatment plants". Anal. Methods. 5 (2): 428. doi:10.1039/c2ay26163d.
- ↑ Balmer, Marianne E.; Buser, Hans-Rudolf; Müller, Markus D.; Poiger, Thomas (2005-02-01). "Occurrence of Some Organic UV Filters in Wastewater, in Surface Waters, and in Fish from Swiss Lakes". Environmental Science & Technology. 39 (4): 953–962. doi:10.1021/es040055r. ISSN 0013-936X.
- ↑ Bachelot, Morgane; Li, Zhi; Munaron, Dominique; Le Gall, Patrik; Casellas, Claude; Fenet, Hélène; Gomez, Elena (2012). "Organic UV filter concentrations in marine mussels from French coastal regions". Science of the Total Environment. 420: 273–9. doi:10.1016/j.scitotenv.2011.12.051. PMID 22330425.
- ↑ Kameda, Yutaka; Kimura, Kumiko; Miyazaki, Motonobu (2011). "Occurrence and profiles of organic sun-blocking agents in surface waters and sediments in Japanese rivers and lakes". Environmental Pollution. 159 (6): 1570–6. doi:10.1016/j.envpol.2011.02.055. PMID 21429641.
- ↑ Vione, D; Calza, P; Galli, F; Fabbri, D; Santoro, V; Medana, C (2015). "The role of direct photolysis and indirect photochemistry in the environmental fate of ethylhexyl methoxy cinnamate (EHMC) in surface waters". Science of the Total Environment. 537: 58–68. doi:10.1016/j.scitotenv.2015.08.002. PMID 26282740.
- 1 2 Sánchez-Quiles, David; Tovar-Sánchez, Antonio (2015). "Are sunscreens a new environmental risk associated with coastal tourism?". Environment International. 83: 158–70. doi:10.1016/j.envint.2015.06.007. PMID 26142925.
- ↑ Chowdhury, Shakhawat; Alhooshani, Khalid; Karanfil, Tanju (2014). "Disinfection byproducts in swimming pool: Occurrences, implications and future needs". Water Research. 53: 68–109. doi:10.1016/j.watres.2014.01.017. PMID 24509344.
- ↑ Sánchez-Quiles, David; Tovar-Sánchez, Antonio (2014). "Sunscreens as a Source of Hydrogen Peroxide Production in Coastal Waters". Environmental Science & Technology. 48 (16): 9037–42. doi:10.1021/es5020696. PMID 25069004.
- ↑ Lesser, Michael P (2006). "OXIDATIVE STRESS IN MARINE ENVIRONMENTS: Biochemistry and Physiological Ecology". Annual Review of Physiology. 68: 253–78. doi:10.1146/annurev.physiol.68.040104.110001. PMID 16460273.
- ↑ Liu, YS (2011). "Photostability of the UV filter benzophenone-3 and its effect on the photodegradation of benzotriazole in water". Environmental Chemistry. 8 (6): 581–8. doi:10.1071/en11068.
- ↑ Santos, A. Joel M; Miranda, Margarida S; Esteves Da Silva, Joaquim C.G (2012). "The degradation products of UV filters in aqueous and chlorinated aqueous solutions". Water Research. 46 (10): 3167–76. doi:10.1016/j.watres.2012.03.057. PMID 22513303.
- ↑ Li, Yingjie; Qiao, Xianliang; Zhou, Chengzhi; Zhang, Ya-nan; Fu, Zhiqiang; Chen, Jingwen (2016). "Photochemical transformation of sunscreen agent benzophenone-3 and its metabolite in surface freshwater and seawater". Chemosphere. 153: 494–9. doi:10.1016/j.chemosphere.2016.03.080. PMID 27035387.
- 1 2 Mao, Liang; Meng, Cui; Zeng, Chao; Ji, Yuefei; Yang, Xi; Gao, Shixiang (2011). "The effect of nitrate, bicarbonate and natural organic matter on the degradation of sunscreen agent p-aminobenzoic acid by simulated solar irradiation". Science of the Total Environment. 409 (24): 5376–81. doi:10.1016/j.scitotenv.2011.09.012. PMID 21975008.
- ↑ Fent, Karl; Zenker, Armin; Rapp, Maja (2010). "Widespread occurrence of estrogenic UV-filters in aquatic ecosystems in Switzerland". Environmental Pollution. 158 (5): 1817–24. doi:10.1016/j.envpol.2009.11.005. PMID 20004505.
- 1 2 3 4 Danovaro, Roberto; Bongiorni, Lucia; Corinaldesi, Cinzia; Giovannelli, Donato; Damiani, Elisabetta; Astolfi, Paola; Greci, Lucedio; Pusceddu, Antonio (1 January 2008). "Sunscreens Cause Coral Bleaching by Promoting Viral Infections". Environmental Health Perspectives. 116 (4): 441–447. doi:10.1289/ehp.10966. JSTOR 40040094. PMC 2291018. PMID 18414624.
External links
- UV filters test - Description of the results and summary - Lenstip.com
- UV filters test - supplement - Introduction - Lenstip.com
- oduction - Lenstip.com
- Sharma, Anežka; Bányiová, Katarína; Babica, Pavel; El Yamani, Naouale; Collins, Andrew Richard; Čupr, Pavel (2017). "Different DNA damage response of cis and trans isomers of commonly used UV filter after the exposure on adult human liver stem cells and human lymphoblastoid cells". Science of the Total Environment. 593-594: 18–26. doi:10.1016/j.scitotenv.2017.03.043. PMID 28340478.