Molybdenum(VI) chloride
 | |
| Names | |
|---|---|
| Other names
molybdenum hexachloride | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
PubChem CID |
|
| UNII | |
| |
| |
| Properties | |
| Cl6Mo | |
| Molar mass | 308.65 g·mol−1 |
| Appearance | black solid |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
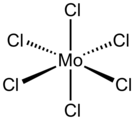
Molybdenum(VI) chloride is the inorganic compound with the formula MoCl6. It is a black diamagnetic solid. The molecules adopt an octahedral structure as seen in tungsten(VI) chloride.[1]
Preparation and reactions
Molybdenum(VI) chloride is prepared from the molybdenum hexafluoride with excess boron trichloride:
- 2 MoF6 + 6 BCl3 → MoCl6 + 6 BF2Cl
It is unstable at room temperature with respect to molybdenum(V) chloride:
- 2 MoCl6 → [MoCl5]2 + Cl2
References
- ↑ Tamadon, Farhad; Seppelt, K., (2012). "The Elusive Halides VCl5, MoCl6, and ReCl6". Angewandte Chemie International Edition. 52: 767–769. doi:10.1002/anie.201207552.
This article is issued from
Wikipedia.
The text is licensed under Creative Commons - Attribution - Sharealike.
Additional terms may apply for the media files.