Triple-stranded DNA
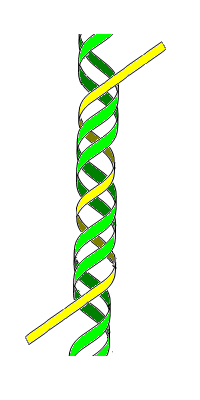
Triple-stranded DNA is a DNA structure in which three oligonucleotides wind around each other and form a triple helix. In this structure, one strand binds to a B-form DNA double helix through Hoogsteen or reversed Hoogsteen hydrogen bonds.
For example, a nucleobase T binds to a Watson–Crick base-pairing of T-A by Hoogsteen hydrogen bonds between an AxT pair (x represents a Hoogsteen base pair). An N-3 protonated cytosine, represented as C+, can also form a base-triplet with a C-G pair through the Hoogsteen base-pairing of an GxC+.
There are two classes of triplex DNA: intermolecular and intramolecular formations. Intermolecular triplex refers to triplex formation between a duplex and a different strand of DNA. The third strand can either be from a neighboring chromosome or a triplex forming oligonucleotide (TFO). Intramolecular triplex DNA is formed from a duplex with homopurine and homopyrimidine strands with mirror repeat symmetry.[1] The level of supercoiling in DNA influences the level of intramolecular triplex formation.[2] There are two different types of intramolecular triplex DNA: H-DNA and H*-DNA. Formation of H-DNA is stabilized under acidic conditions and in the presence of divalent cations such as Mg2+. In this conformation, the homopyrimidine strand in the duplex bends back to bind to the purine strand in a parallel fashion. The base triads used to stabilize this conformation are T-A-*T and C-G*C+. The cytosine in this base triad needs to be protonated in order to form this intramolecular triple helix, which is why this conformation is stabilized under acidic conditions.[3] H*-DNA has favorable formation conditions at neutral pH and in the presence of divalent cations.[4] This intramolecular conformation is formed from the binding of the homopurine and purine strand of the duplex in an antiparallel fashion. It is stabilized by T-A*A and C-G*G base triplets.[5][6]
History
Triple-stranded DNA was a common hypothesis in the 1950s when scientists were struggling to discover DNA's true structural form. Watson and Crick (who later won the Nobel Prize for their double-helix model) originally considered a triple-helix model, as did Pauling and Corey, who published a proposal for their triple-helix model in 1953, as well as fellow scientist Fraser. However, Watson and Crick soon identified several problems with these models:
- Negatively charged phosphates near the axis repel each other, leaving the question of how the three-chain structure stays together.
- In a triple-helix model (specifically Pauling and Corey's model), some of the van der Waals distances appear to be too small.
Fraser's model differed from Pauling and Corey's in that in his model the phosphates are on the outside and the bases are on the inside, linked together by hydrogen bonds. However, Watson and Crick found Fraser's model to be too ill-defined to comment specifically on its inadequacies.
Triple-stranded DNA was also described in 1957, when it was thought to occur in only one in vivo biological process: as an intermediate product during the action of the E. coli recombination enzyme RecA. Its role in that process is not understood.
Using nucleic acid segments that bind to the DNA duplexes to form triple strands as a way of regulating gene expression is under separate investigation by biotechnology companies and Yale University.
Function
Triple-stranded DNA is implicated in the regulation of several genes. Once, the c-myc gene was extensively mutated to examine the role that triplex DNA structure, versus linear sequence, plays in gene regulation. A c-myc promoter element, termed the nuclease-sensitive element or NSE, can form tandem intramolecular triplexes of the H-DNA type and has a repeating sequence motif (ACCCTCCCC)4. The NSE when mutated was examined for transcriptional activity and for intra- and intermolecular triplex forming ability. The transcriptional activity of mutant NSEs can be predicted by the element's ability to form H-DNA and not by repeat number, position, or the number of mutant base pairs. DNA may therefore be a dynamic participant in the transcription of the c-myc gene.[7]
TFOs are triplex forming molecules that bind to the major groove of the double stranded DNA to form intramolecular triplex DNA structures. TFOs bind specifically to homopurine-homopyrimidine regions. TFOs can inhibit transcription by actively competing with the binding of the transcription factor. Because of the high specificity of the triplex forming molecule, TFOs have been of interest in inhibiting transcription of genes. By using highly specific DNA segments to target TFO regions, expression of genes can be controlled.[8] This application has novel implications in site-specific mutagenesis and gene therapy. The observed inhibition of transcription can also have negative health effects like its role in the recessive, autosomal gene for Friedreich’s Ataxia.[9] In Fredrick’s Ataxia, triplex DNA formation impairs the expression of intron 1 of the FXN gene. This results in the degeneration of the nervous system and spinal cord, impairing the movement of the limbs.[10]
TFOs are promising gene-drugs that can be used in an anti-gene strategy. They attempt to modulate gene activity in vivo. Chemical modifications of TFO are known. In peptide nucleic acid (PNA), the sugar-phosphate backbone is replaced with a protein-like backbone. PNAs form P-loops while interacting with duplex DNA, forming triplex with one DNA strand displacing the other. Very unusual recombination or parallel triplexes, or R-DNA, have been assumed to form under RecA protein in the course of homologous recombination.[11]
References
- ↑ Ussery, D.W.; Sinden R.R. (1993). "Environmental Influences on the in Vivo Level of Intramolecular Triplex DNA in Escherichia coli". Biochemistry. 32: 6206–6213. doi:10.1021/bi00075a013.
- ↑ Dayn, A.; Samadashwily, G. M.; Mirkin, S. M. (1992). "Intramolecular DNA triplexes: Unusual sequence requirements and influence on DNA polymerization" (PDF). Biochemistry. 89: 11406–11410. Bibcode:1992PNAS...8911406D. doi:10.1073/pnas.89.23.11406. PMC 50559. PMID 1454828.
- ↑ Lyamichev, V. I.; Mirkin, S. M.; Frank-Kamenetskii, M. D. (1986). "Structures of homopurine-homopyrimidine tract in superhelical DNA". Biomol Struct Dyn. 3 (667–669): 667–9. doi:10.1080/07391102.1986.10508454. PMID 3271043.
- ↑ Dayn, A.; Samadashwily, G. M.; Mirkin, S. M. (1992). "Intramolecular DNA triplexes: Unusual sequence requirements and influence on DNA polymerization" (PDF). Biochemistry. 89: 11406–11410. Bibcode:1992PNAS...8911406D. doi:10.1073/pnas.89.23.11406. PMC 50559. PMID 1454828.
- ↑ Ussery, D.W.; Sinden R.R. (1993). "Environmental Influences on the in Vivo Level of Intramolecular Triplex DNA in Escherichia coli". Biochemistry. 32: 6206–6213. doi:10.1021/bi00075a013.
- ↑ Lyamichev, V. I.; Mirkin, S. M.; Frank-Kamenetskii, M. D. (1986). "Structures of homopurine-homopyrimidine tract in superhelical DNA". Biomol Struct Dyn. 3 (667–669): 667–9. doi:10.1080/07391102.1986.10508454. PMID 3271043.
- ↑ Firulli, A.B.; Maibenco, D.C.; Kinniburgh, A.J. (1994). "Triplex Forming Ability of a c-myc Promoter Element Predicts Promoter Strength". Archives of Biochemistry and Biophysics. 310 (1): 236–42. doi:10.1006/abbi.1994.1162. PMID 8161210.
- ↑ Faria, M.; Wood, C.D; Perrouault, L.; Nelson, J.S; Winter, A.; White, M.R.H.; Helene, C.; Giovannangeli, C. (2000). "Targeted inhibition of transcription elongation in cells mediated by triplex-forming oligonucleotides". PNAS. 97: 3862–3867. Bibcode:2000PNAS...97.3862F. doi:10.1073/pnas.97.8.3862. PMC 18107.
- ↑ Sakamoto N.; Chastain, P.D; Parniewski, P.; Ohshima, K.; Pandolfo, M.; Griffith, J.D; Wells, R.D. (1999). "Sticky DNA: Self-Association Properties of Long GAA·TTC Repeats in R·R·Y Triplex Structures from Friedreich's Ataxia". Molecular Cell. 3 (4): 465–475. doi:10.1016/s1097-2765(00)80474-8. PMID 10230399.
- ↑ Bacolla, A.; Wells, R.D. (2009). "Non-B DNA Conformations as Determinants of Mutagenesis and Human Disease". Human Carcinogenisis. 48: 273–285. doi:10.1002/mc.20507. PMID 19306308.
- ↑ Frank-Kamenetskii, M. D.; Mirkin, S. M. (1995-01-01). "Triplex DNA structures". Annual Review of Biochemistry. 64: 65–95. doi:10.1146/annurev.bi.64.070195.000433. ISSN 0066-4154. PMID 7574496.
Sources
- Rich, Alexander (1993). "DNA comes in many forms". Gene. 135 (1–2): 99–109. doi:10.1016/0378-1119(93)90054-7. PMID 8276285.
- Soyfer, Valery N.; Potaman, Vladimir N. (1995). Triple-Helical Nucleic Acids. New York: Springer. ISBN 978-0-387-94495-1.
- Mills, Martin; Arimondo, Paola B.; Lacroix, Laurent; Garestier, Thérèse; Hélène, Claude; Klump, Horst; Mergny, Jean-Louis (1999). "Energetics of strand-displacement reactions in triple helices: A spectroscopic study". Journal of Molecular Biology. 291 (5): 1035–54. doi:10.1006/jmbi.1999.3014. PMID 10518941.
- Watson, J. D.; Crick, F. H. C. (1953). "Molecular Structure of Nucleic Acids: A Structure for Deoxyribose Nucleic Acid". Nature. 171 (4356): 737–8. Bibcode:1953Natur.171..737W. doi:10.1038/171737a0. PMID 13054692.