Soap scum
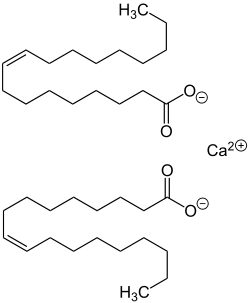
| Calcium oleate, Calcium palmitate, and Calcium stearate |
 |
 |
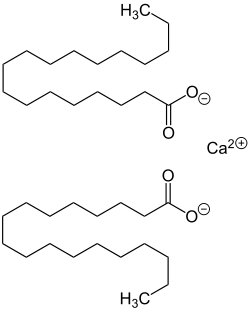 |
Soap scum or lime soap is the white solid composed of calcium stearate, magnesium stearate, and similar insoluble compounds that results from the addition of soap and other anionic surfactants to hard water. Hard water contains calcium and magnesium ions, which react with the surfactant anion to give metallic or lime soaps:
- 2 C17H35COO−Na+ + Ca2+ → (C17H35COO)2Ca + 2 Na+
In this reaction, the sodium cation in soap is replaced by calcium to form calcium stearate.
Lime soaps build deposits on fibres, washing machines, and sinks. Synthetic surfactants are less susceptible to the effects of hard water. Most detergents contain builders that prevent the formation of lime soaps.
Soap scum on vinyl shower curtains may have a rich microbial biofilm, containing potentially pathogenic bacteria.[1][2]
See also
References
- ↑ Shower Curtains May Harbor Harmful Microbes, American Society for Microbiology
- ↑ Kelley, Scott; Theisen, Ulrike (July 2004). "Molecular Analysis of Shower Curtain Biofilm Microbes". Applied and Environmental Microbiology. American Society for Microbiology. 70 (7): 4187–4192. CiteSeerX 10.1.1.325.1112. doi:10.1128/AEM.70.7.4187-4192.2004.