Sextuple bond
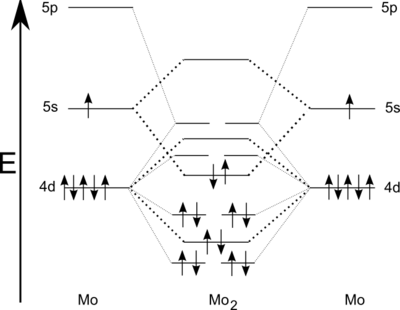
A sextuple bond is a type of covalent bond involving 12 bonding electrons and in which the bond order is 6. The only known molecules with true sextuple bonds are the diatomic dimolybdenum (Mo2) and ditungsten (W2), which exist in the gaseous phase and have boiling points of 4,639 °C (8,382 °F) and 5,930 °C (10,710 °F). There is strong evidence to believe that there is no element with atomic number below about 100 that can form a bond with a greater order than 6 between its atoms,[1] but the question of possibility of such a bond between two atoms of different elements remains open.
Dimolybdenum and ditungsten
Dimolybdenum (Mo2) can be observed in the gas phase at low temperatures (7 K) by a laser evaporation technique using molybdenum sheet with, for instance, near-infrared spectroscopy or UV spectroscopy.[2] Like dichromium, a singlet state is expected from dimolybdenum.[3] Higher bond order is reflected in shorter bond length of 194 pm.
Other molecules
Although diatomic Cr2 and U2 have formal structures with twelve-electron bonds, their effective bond orders derived from quantum chemistry calculations are less than 5 (which would be quintuple bonds).[1]
References
- 1 2 Roos, Björn O.; Borin, Antonio C.; Laura Gagliardi (2007). "Reaching the Maximum Multiplicity of the Covalent Chemical Bond". Angew. Chem. Int. Ed. 46 (9): 1469–72. doi:10.1002/anie.200603600. PMID 17225237.
- ↑ Kraus, D.; Lorenz, M.; Bondybey, V. E. (2001). "On the dimers of the VIB group: a new NIR electronic state of Mo2". Phys. Chem. Comm. 4 (10): 44–48. doi:10.1039/b104063b.
- ↑ Merino, Gabriel; Donald, Kelling J.; D’Acchioli, Jason S.; Hoffmann, Roald (2007). "The Many Ways To Have a Quintuple Bond". J. Am. Chem. Soc. 129 (49): 15295–15302. doi:10.1021/ja075454b. PMID 18004851.
Further reading
- Bursten, Bruce E.; Cotton, F. Albert; B. Hall, Michael (1980). "Dimolybdenum: nature of the sextuple bond". J. Am. Chem. Soc. 102 (20): 6348. doi:10.1021/ja00540a034.
- Borin, Antonio Carlos; Gobbo, João Paulo; Roos, Björn O. (2008). "A theoretical study of the binding and electronic spectrum of the Mo2 molecule". Chem. Phys. 343 (2–3): 210. Bibcode:2008CP....343..210B. doi:10.1016/j.chemphys.2007.05.028.
- Goodgame, Marvin M.; Goddard, William A., III (1981). "The "sextuple" bond of chromium dimer". J. Phys. Chem. 85 (3): 215. doi:10.1021/j150603a001.
- Chisholm, M. H. (Feb 2007). "Metal to metal multiple bonds in ordered assemblies" (Free full text). PNAS. 104 (8): 2563–70. Bibcode:2007PNAS..104.2563C. doi:10.1073/pnas.0610364104. PMC 1815223. PMID 17299047.
- Norman, Joe G., Jr.; Ryan, P. Barry (1980). "Metal–metal bond energies in diatomic molybdenum, octachloromolybdate (Mo
2Cl4−
8), and molybdenum formate (Mo
2(O
2CH)
4)". J. Comp. Chem. 1 (1): 59–63. doi:10.1002/jcc.540010107. - Atha, P. M.; Hillier, I. H.; Guest, M. F. (1980). "Electron correlation and the nature of the sextuple bond in the dimolybdenum molecule". Chem. Phys. Lett. 75 (1): 84–86. Bibcode:1980CPL....75...84A. doi:10.1016/0009-2614(80)80469-6.
- Wood, Carol; Doran, Mark; Hillier, Ian H.; Guest, Martyn F. (1980). "Theoretical study of the electronic structure of the transition metal dimers, discandium, dichromium, dimolybdenum, and dinickel". Faraday Symp. R. Soc. Chem. 14 (Diatomic Metal–Metal Clusters): 159–169. doi:10.1039/fs9801400159.