Phi bond
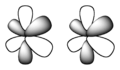

Suitably-aligned f atomic orbitals can overlap to form a φ molecular orbital (a φ bond)
In chemistry, phi bonds (φ bonds) are covalent chemical bonds, where six lobes of one involved atomic orbital overlap six lobes of the other involved atomic orbital. This overlap leads to the formation of a bonding molecular orbital with three nodal planes which contain the internuclear axis and go through both atoms.
The Greek letter φ in their name refers to f orbitals, since the orbital symmetry of the φ bond is the same as that of the usual (6-lobed) type of f orbital when seen down the bond axis.
There is as of 2005 only one known example of a molecule purported to contain a phi bond (a U−U bond, in the molecule U2).[1]
References
- ↑ Gagliardi, Laura; Roos, Björn O. (2005). "Quantum chemical calculations show that the uranium molecule U2 has a quintuple bond". Nature. 433 (7028): 848–851. Bibcode:2005Natur.433..848G. doi:10.1038/nature03249. PMID 15729337.
This article is issued from
Wikipedia.
The text is licensed under Creative Commons - Attribution - Sharealike.
Additional terms may apply for the media files.