Semisynthesis
Semisynthesis or partial chemical synthesis is a type of chemical synthesis that uses chemical compounds isolated from natural sources (e.g., microbial cell cultures or plant material) as the starting materials to produce other novel compounds with distinct chemical and medicinal properties. These novel compounds are generally high molecular weight or have complex molecular structure, more so than those produced by total synthesis from simple starting materials. Semisynthesis is a means of preparing many medicines more cheaply than if by total synthesis, because fewer chemical steps are necessary.
Overview
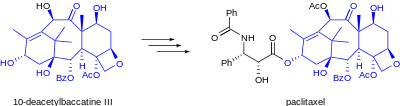
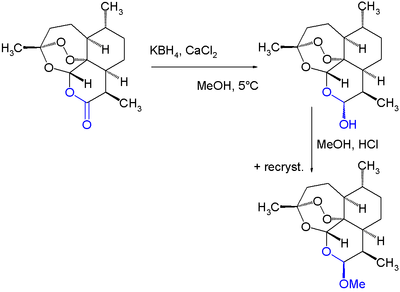
Drugs derived from natural sources are usually produced by isolation from the natural source, or, as described here, by semisynthesis from such an isolated agent. From the viewpoint of chemical synthesis, living organisms are remarkable chemical factories, capable of producing structurally complex chemical compounds with ease by biosynthesis. In contrast, engineered chemical synthesis is necessarily simpler, with a lower chemical diversity in each reaction, than the incredibly diverse biosynthesis pathways that are crucial to life. As a result, certain functional groups are much easier to prepare via engineered synthesis than others – for example, acetylation – where certain biosynthetic pathways can generate groups and structures with minimal economic input that would be prohibitive via total synthesis. Plants, animals, fungi, and bacteria are all used as sources for these tricky precursor molecules, including the use of bioreactors at the meeting point between engineered and biological chemical synthesis.
Semisynthesis, when used in drug discovery, aims to retain the sought-after medicinal activity while altering other molecule characteristics – for instance, those affecting its adverse events or its oral bioavailability – in a few chemical steps. In this regard, semisynthesis stands in contrast with the approach of total synthesis, where the aim is to arrive at a target molecule beginning with low-molecular-weight, inexpensive starting materials – often petrochemicals or minerals.[3] While there is no hard-and-fast division between total synthesis and semisynthesis – rather differing in the degree of engineered synthesis used – in practice many commodity precursor molecules with complex or fragile functional groups are much cheaper to extract from an organism than to prepare from simple precursors only. Hence, methods of semisynthesis are applied when a needed precursor molecule is too structurally complex, too costly, or too difficult to produce by total synthesis.
Examples of practical application of the use of semisynthesis include in the groundbreaking historic case of the isolation of the antibiotic chlortetracycline, and the semisyntheses of the further novel antibiotics tetracycline, doxycycline, and tigecycline.[4][5] Further examples of semisynthesis include the early commercial production of anti-cancer agent paclitaxel from 10-deacetylbaccatin isolated from the needles of Taxus baccata (European yew),[1] the preparation of LSD from ergotamine isolated from fungal cultures of ergot, and the semisynthesis of the antimalarial drug artemether from naturally occurring artemisinin.[2] As the field of synthetic chemistry advances, certain transformations become cheaper or easier, and the economics of a semisynthetic route may become less favorable.[3]
See also
- Drug discovery
- Drug development
- Production of cephalopsporins from 7-ACA
- Production of penicillins from 6-APA
- Production of steroids from 16-DPA
References
- 1 2 Goodman, Jordan; Walsh, Vivien (5 March 2001). The Story of Taxol: Nature and Politics in the Pursuit of an Anti-Cancer Drug. Cambridge University Press. pp. 100f. ISBN 978-0-521-56123-5.
- 1 2 Boehm M, Fuenfschilling PC, Krieger M, Kuesters E, Struber, F (2007). "An Improved Manufacturing Process for the Antimalaria Drug Coartem. Part I". Org. Process Res. Dev. 11 (3): 336–340. doi:10.1021/op0602425.
- 1 2 "Welcome to Chemistry World". Chemistry World.
- ↑ Nelson ML, Levy SB (2011). "The History of the Tetracyclines". Annals of the New York Academy of Sciences. 1241 (December): 17–32. Bibcode:2011NYASA1241...17N. doi:10.1111/j.1749-6632.2011.06354.x. PMID 22191524.
- ↑ Liu F, Myers, AG (2016). "Development of a Platform for the Discovery and Practical Synthesis of New Tetracycline Antibiotics" (PDF). Current Opinion in Chemical Biology. 32: 48–57. doi:10.1016/j.cbpa.2016.03.011.