Transition metal alkyne complex
In organometallic chemistry, a transition metal alkyne complex is a coordination compound containing one or more alkyne ligands. Such compounds are intermediates in many catalytic reactions that convert alkynes to other organic products, e.g. hydrogenation and trimerization.[1]
Synthesis
Transition metal alkyne complexes are often formed by the displacement of labile ligands by the alkyne. For example, a variety of cobalt-alkyne complexes may be formed by reaction of the alkyne with dicobalt octacarbonyl.[2]
- Co2(CO)8 + R2C2 → Co2(C2R2)(CO)6 + 2 CO
Many alkyne complexes are produced by reduction of metal halides, e.g. titanocene dichloride and bis(triphenylphosphine)platinum dichloride in the presence of the alkyne:
- Cp2TiCl2 + C2R2 + Mg → Cp2Ti(C2R2) + MgCl2
Structure and Bonding
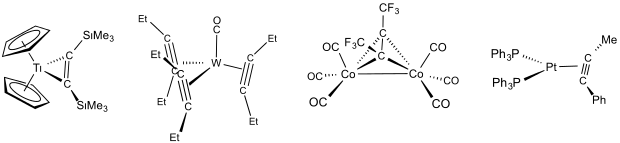
The coordination of alkynes to transition metals is similar to that of alkenes. The bonding is described by the Dewar-Chatt-Duncanson model. Upon complexation the C-C bond elogates and the alkynyl carbon bends away from 180º. For example, in the phenylpropyne complex Pt(PPh3)2(C2)Ph(Me), the C-C distance is 1.277(25) vs 1.20 Å for a typical alkyne. The C-C-C angle distorts 40° from linearity.[3] Because the bending induced by complexation, strained alkynes such as cycloheptyne and cyclooctyne are stabilized by complexation.[4]
In the IR spectra, the C-C vibration of alkynes, which occurs near 2300 cm−1, shifts upon complexation to around 1800 cm−1, indicating a weakening of the C-C bond.
η2-coordination to a single metal center
When bonded side-on to a single metal atom, an alkyne serves as a dihapto usually two-electron donor. For early metal complexes, e.g., Cp2Ti(C2R2), strong π-backbonding into one of the π* antibonding orbitals of the alkyne is indicated. This complex is described as a metallacyclopropene derivative of Ti(IV). For late transition metal complexes, e.g., Pt(PPh3)2(MeC2Ph), the π-backbonding is less prominent, and the complex is assigned oxidation state (0).[5][6]
In some complexes, the alkyne is classified as a four-electron donor. In these cases, both pairs of pi-electrons donate to the metal. This kind of bonding was first implicated in complexes of the type W(CO)(R2C2)3.[7]
η2, η2-coordination bridging two metal centers
Because alkynes have two π bonds, alkynes can form stable complexes in which they bridge two metal centers. The alkyne donates a total of four electrons, with two electrons donated to each of the metals. And example of a complex with this bonding scheme is η2-diphenylacetylene-(hexacarbonyl)dicobalt(0).[6]
Benzyne complexes
Transition metal benzyne complexes represent a special case of alkyne complexes since the free benzynes are not stable in the absence of the metal.[8]
Applications
Metal alkyne complexes are intermediates in the semihydrogenation of alkynes to alkenes:
- C2R2 + H2 → cis-C2R2H2
This transformation is conducted on a large scale in refineries, which unintentionally produce acetylene during the production of ethylene. It is also useful in the preparation of fine chemicals. Semihydrogenation affords cis alkenes.[9]
Metal-alkyne complexes are also intermediates in the metal-catalyzed trimerization and tetramerizations. Cyclooctatetraene is produced from acetylene via the intermediacy of metal alkyne complexes. Variant of this reaction are exploited for the synthesis of substituted pyridines.
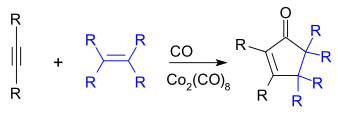
The Pauson-Khand reaction provides a route to cyclopentenones via the intermediacy of cobalt-alkyne complexes.
With the shift away from coal-based (acetylene) to petroleum-based feedstocks (olefins), catalytic reactions with alkynes are not widely practiced industrially. Acrylic acid was once prepared by the hydrocarboxylation of acetylene:[10]
- C2H2 + H2O + CO → H2C=CHCO2H
References
- ↑ Elschenbroich, C. ”Organometallics” 2006 Wiley-VCH: Weinheim. ISBN 3-527-29390-6.
- ↑ Kemmitt, R. D. W.; Russell, D. R.; "Cobalt" in Comprehensive Organometallic Chemistry I; Abel, E.W.; Stone, F.G.A.; Wilkinson, G. eds., 1982, Pergamon Press, Oxford. ISBN 0-08-025269-9
- ↑ William Davies, B.; C. Payne, N., "Studies on metal-acetylene complexes: V. Crystal and molecular structure of bis(triphenylphosphine)(1-phenylpropyne)platinum(0), [P(C6H5)3]2(C6H5CCCH3)Pt0" J. Organomet. Chem. 1975, volume 99, pp. 315. doi:10.1016/S0022-328X(00)88462-4
- ↑ Bennett, Martin A.; Schwemlein, Heinz P. (1989). "Metal Complexes of Small Cycloalkynes and Arynes". Angew. Chem. Int. Ed. Engl. 28 (10): 1296–1320. doi:10.1002/anie.198912961.
- ↑ Hill, A.F. Organotransition Metal Chemistry, 2002, Royal Society of Chemistry, ISBN 0-471-28163-8.
- 1 2 Crabtree, R. H. Comprehensive Organometallic Chemistry V, 2009, John Wiley & Sons, Inc. ISBN 978-0-470-25762-3
- ↑ Joseph L. Templeton "Four-Electron Alkyne Ligands in Molybdenum(II) and Tungsten(II) Complexes" Advances in Organometallic Chemistry 1989, Volume 29, Pages 1–100.doi:10.1016/S0065-3055(08)60352-4
- ↑ William M. Jones, Jerzy Klosin "Transition-Metal Complexes of Arynes, Strained Cyclic Alkynes, and Strained Cyclic Cumulenes" Advances in Organometallic Chemistry 1998, Volume 42, Pages 147–221. doi:10.1016/S0065-3055(08)60543-2
- ↑ Michaelides, I. N.; Dixon, D. J. (2013). "Catalytic Stereoselective Semihydrogenation of Alkynes to E-Alkenes". Angew. Chem. Int. Ed. 52: 806–808. doi:10.1002/anie.201208120.
- ↑ W. Bertleff; M. Roeper; X. Sava, "Carbonylation", Ullmann's Encyclopedia of Industrial Chemistry, Weinheim: Wiley-VCH, doi:10.1002/14356007.a05_217