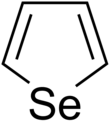
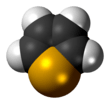
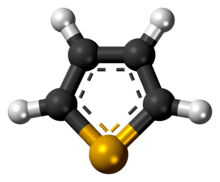
Selenophene
| |||
 | |||
| Identifiers | |||
|---|---|---|---|
| 103223 | |||
| ChEBI | |||
| ChemSpider | |||
| ECHA InfoCard | 100.157.009 | ||
| 100994 | |||
PubChem CID |
|||
| |||
| |||
| Properties | |||
| C4H4Se | |||
| Molar mass | 131.05 g·mol−1 | ||
| Density | 1.52 | ||
| Melting point | −38 °C (−36 °F; 235 K) | ||
| Boiling point | 110 °C (230 °F; 383 K) | ||
Refractive index (nD) |
1.58 | ||
| Hazards | |||
| GHS pictograms |     | ||
| GHS signal word | Danger | ||
| H225, H301, H331, H373, H400, H410 | |||
| P210, P233, P240, P241, P242, P243, P260, P261, P264, P270, P271, P273, P280, P301+310, P303+361+353, P304+340, P311, P314, P321, P330, P370+378, P391, P403+233, P403+235, P405 | |||
| Related compounds | |||
Related more saturated |
selenolane 2-selenolene 3-selenolene | ||
Related compounds |
furan thiophene tellurophene | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
Selenophene is an unsaturated organic compound containing a five member ring with selenium with formula C4H4Se. It is a metallole with reduced aromatic character compared to thiophene.
Nomenclature
Atoms in selenophene are numbered starting from "1" for selenium, increasing clockwise around the ring. Substituents may occur on carbon atoms 2 to 4. Related compounds are the saturated selenolane C4H8Se, and rings with only one double bond 2-selenolene and 3-selenolene.[1] Extra bonds can also form to the selenium atom, and these will be prefixed with 1- or 1,1- eg selenophene 1,1-dioxide.[2]
Production
Although Ida Foa claimed to have made selenophene in 1909, the first confirmed production was by Mazza and Solazzo in 1927. They heated acetylene and selenium together at about 300°. The selenium burst into flame, and up to 15% selenophene was formed, along with selenonaphthene.[1] Another way to make it is from furan heated with hydrogen selenide and aluminium at 400°C.[3]
Substituted selenophenes can be made using a Fiesselman procedure in which a β-chloro-aldehyde reacts with sodium selenide, and then ethyl bromoacetate.[3]
Properties
The selenophene molecule is flat and aromatic.[3] Being aromatic it undergoes electrophilic substitution reactions at the -2 or -2,5 positions.[3] These reactions are slower than that of furan, but faster than thiophene.[3]
References
- 1 2 Hartough, H. D. (2009). Thiophene and Its Derivatives. John Wiley & Sons. ISBN 9780470188026.
- ↑ Pelkey, E. T. (2008). Katritzky, Alan R.; Ramsden, Christopher A.; Scriven, Eric F. V.; Taylor, Richard J. K., eds. Comprehensive Heterocyclic Chemistry III. Oxford: Elsevier. pp. 975–1006. ISBN 9780080449920.
- 1 2 3 4 5 Eicher, Theophil; Hauptmann, Siegfried; Speicher, Andreas (2013). The Chemistry of Heterocycles: Structures, Reactions, Synthesis, and Applications. John Wiley & Sons. pp. 69–70. ISBN 9783527669868.