Saline water
| Part of a series on |
| Water salinity |
|---|
 |
| Salinity levels |
|
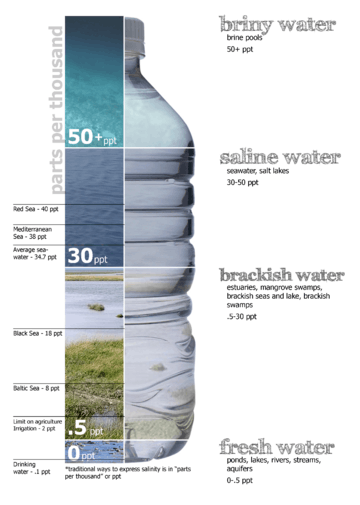
Fresh water (< 0.05%) Brackish water (0.05–3%) Saline water (3–5%) Brine (> 5%) |
| Bodies of water |
| Seawater • Salt lake • Hypersaline lake • Salt pan • Brine pool • Bodies by salinity |
Saline water (more commonly known as salt water) is water that contains a high concentration of dissolved salts (mainly NaCl). The salt concentration is usually expressed in parts per thousand (permille, ‰) or parts per million (ppm). The United States Geological Survey classifies saline water in three salinity categories. Salt concentration in slightly saline water is around 1,000 to 3,000 ppm (0.1–0.3%), in moderately saline water 3,000 to 10,000 ppm (0.3–1%) and in highly saline water 10,000 to 35,000 ppm (1–3.5%). Seawater has a salinity of roughly 35,000 ppm, equivalent to 35 grams of salt per one liter (or kilogram) of water. The saturation level is dependent on the temperature of the water. At 20 °C one milliliter of water can dissolve about 0.357 grams of salt; a concentration of 26.3%. At boiling (100 °C) the amount that can be dissolved in one milliliter of water increases to about 0.391 grams or 28.1% saline solution.[1]
Some industries make use of saline water, such as mining and thermo-electric power.
Properties
| NaCl, wt% | Freezing point (°C) | Density[lower-alpha 1] (g/cm3) | Refractive index[lower-alpha 2] at 589 nm | Viscosity[lower-alpha 3] (cP ) |
|---|---|---|---|---|
| 0 | 0 | 0.99984 | 1.3330 | 1.002 |
| 0.5 | −0.3 | 1.0018 | 1.3339 | 1.011 |
| 1 | −0.59 | 1.0053 | 1.3347 | 1.02 |
| 2 | −1.19 | 1.0125 | 1.3365 | 1.036 |
| 3 | −1.79 | 1.0196 | 1.3383 | 1.052 |
| 4 | −2.41 | 1.0268 | 1.3400 | 1.068 |
| 5 | −3.05 | 1.0340 | 1.3418 | 1.085 |
| 6 | −3.7 | 1.0413 | 1.3435 | 1.104 |
| 7 | −4.38 | 1.0486 | 1.3453 | 1.124 |
| 8 | −5.08 | 1.0559 | 1.3470 | 1.145 |
| 9 | −5.81 | 1.0633 | 1.3488 | 1.168 |
| 10 | −6.56 | 1.0707 | 1.3505 | 1.193 |
| 12 | −8.18 | 1.0857 | 1.3541 | 1.25 |
| 14 | −9.94 | 1.1008 | 1.3576 | 1.317 |
| 16 | −11.89 | 1.1162 | 1.3612 | 1.388 |
| 18 | −14.04 | 1.1319 | 1.3648 | 1.463 |
| 20 | −16.46 | 1.1478 | 1.3684 | 1.557 |
| 26 | −19.18 | 1.193 | 1.3721 | 1.676 |
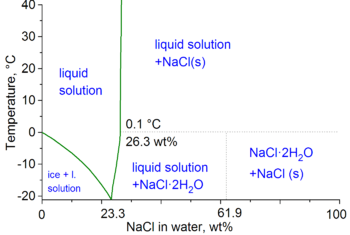
At 100 °C (373.15 K, 212 °F), saturated sodium chloride brine is about 28% salt by weight. At 0 °C (273.15 K, 32 °F), brine can only hold about 26% salt.[3]
The thermal conductivity of seawater (3.5% dissolved salt by weight) is 0.6 W/mK at 25 °C.[4] The thermal conductivity decreases with increasing salinity and increases with increasing temperature. The graphs and online calculations in this page plot thermal conductivity for varying salinity and temperature:[5]
The salt content can be determined with a salinometer.
Density ρ of brine at various concentrations and temperatures can be approximated with a linear equation:[6]
where the values of an are:
| Weight % | a2 | a3 |
|---|---|---|
| 5 | 0.043 | 72.60 |
| 10 | 0.039 | 73.72 |
| 15 | 0.035 | 74.86 |
| 20 | 0.032 | 76.21 |
| 25 | 0.030 | 77.85 |
Electrolysis
About four percent of hydrogen gas produced worldwide is created by electrolysis. The majority of this hydrogen produced through electrolysis is a side product in the production of chlorine.
- 2 NaCl(aq) + 2 H2O(l) → 2 NaOH(aq) + H2(g) + Cl2(g)
See also
References
- ↑ "Solubility". Fundamentals of Chemistry. 2000. Retrieved 6 November 2014.
- ↑ Lide, D. R., ed. (2005). CRC Handbook of Chemistry and Physics (86th ed.). Boca Raton (FL): CRC Press. pp. 8–71, 8–116. ISBN 0-8493-0486-5.
- ↑ CRC Handbook of Chemistry and Physics, 63rd Edition 1982-1983.
- ↑ web.mit.edu
- ↑ "Thermal conductivity of seawater and its concentrates". twt.mpei.ac.ru. Retrieved 2018-02-16.
- ↑ Dittman, Gerald L. (February 16, 1977). "Calculation of Brine Properties". Lawrence Livermore Laboratories. Livermore CA.
External links
![]()