Right-to-try law
Right-to-try laws are U.S. state and Federal laws that were created to let terminally ill patients try experimental therapies (drugs, biologics, devices) that have completed Phase I testing but have not been approved by the Food and Drug Administration (FDA). The value of these laws has been questioned on multiple grounds, including the fact that pharmaceutical manufacturers would have no obligation to provide the therapies being sought.[1]
States with right-to-try laws
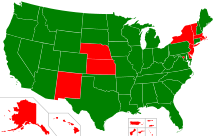
In May 2014, Colorado became the first state to pass a right-to-try law.[3] As of August 2018, 41 states have enacted such laws: Alabama, Alaska, Arizona, Arkansas, California, Colorado, Connecticut, Florida, Georgia, Idaho, Iowa, Illinois, Indiana, Kentucky, Louisiana, Maine, Maryland, Michigan, Minnesota, Mississippi, Missouri, Montana, Nebraska, Nevada, New Hampshire, North Carolina, North Dakota, Ohio, Oklahoma, Oregon, Pennsylvania, South Carolina, South Dakota, Tennessee, Texas, Utah, Virginia, Washington, West Virginia, Wisconsin, and Wyoming.[2][4][5][6]
Federal Right to Try Law
In January 2017, a federal right to try bill was introduced in the Senate by Republican Ron Johnson of Wisconsin.[7] Johnson was able to pass his bill in the Senate on August 3, 2017 in a unanimous consent motion.[8] Johnson had threatened to hold up a Senate vote on the FDA Reauthorization Act of 2017 (FDARA), a must-pass piece of legislation that allows the FDA to operate, if an amendment on right to try was not added to that bill. Johnson agreed to drop a hold on FDARA in exchange for a unanimous consent motion.[9] A companion House bill was introduced in February 2017; the following month it was referred to the House Subcommittee on Crime, Terrorism, Homeland Security, and Investigations.[10] On March 21, 2018, the House of Representatives passed a right to try bill, sending it to the Senate for consideration.[11] On May 22, the Senate passed the bill, and it was then sent to the President's desk for his signature.[12] On May 30, President Trump signed the bill into law.[13]
Proponents
The chief advocate of right-to-try laws is the Goldwater Institute, a libertarian think tank based in Arizona, which created the legislation template on which the state laws are modeled.[14] Kurt Altman, national policy adviser for the Institute, has said that right-to-try laws return control of medical decisions "back to a local level".[15] Other proponents include patients and their families, as well as patient advocate groups.[16] Supporters of these laws sometimes describe them as "Dallas Buyers Club" bills, a reference to a movie about an American man with AIDS who smuggled unapproved treatments from foreign countries to fellow patients.[1] Some have likened the efforts of terminally ill patients to procure unapproved drugs in development to those of ACT-UP and other AIDS organizations of the 1980s.[17]
One ethical argument for the right to try unapproved treatments is that if patients have the right to die through physician-assisted suicide or voluntary euthanasia, they should also be afforded the right to try.[18]
Critics
Bioethicists and other scholars have questioned the extent to which right-to-try laws will actually benefit patients. Jonathan Darrow, Arthur Caplan, Alta Charo, Rebecca Dresser, Alison Bateman-House and others have pointed out that the laws do not require physicians to prescribe experimental therapies, do not require insurance companies to pay for them, and do not require manufacturers to provide them.[19][20] Because the laws do not actually provide a right to receive experimental therapies, they could be considered toothless legislation that offers only false hope to dying people.[21][22] Even if the laws work as intended, they would be problematic to critics. Because the laws require only that drugs have completed the first of three phases of clinical testing, there is no data on the efficacy of the drugs, especially in very sick people. There is also no safety data on how they would affect very sick people. This makes informed consent on the part of the patient more difficult, because informed consent entails, first, knowledge of the pros and cons of a proposed treatment and then a decision made in light of those pros and cons.[23] Some states' right-to-try laws also put patients at risk of losing hospice or home health care,[24] and the costs surrounding treatment can be prohibitive, something right-to-try laws do not fix. Bioethicist Alta Charo called the laws "a simplistic way of going after much more complicated issues."[25]
Medical and health experts have also voiced concerns. If the laws were to grant patients access to unapproved drugs, they could hasten death or cause increased suffering.[26] Peter Temin wrote that "there is always a chance that any given drug will fail to cure a condition or will induce an adverse reaction," such as becoming sick, or sicker, or even dying.[27] Drugs that are not fully studied may lead to more adverse reactions in patients. The laws reduce FDA oversight of drug regulation.[28] Another criticism is that state right-to-try laws may be unconstitutional, because they involve states regulating medicine despite federal legislation that regulates the interstate marketing of medicine.[29] Various authors have predicted that right-to-try laws would be struck down if they were taken to court.[30][31] A 2014 paper in JAMA Internal Medicine argued that right to try laws "seem likely to be futile."[32]
In April 2017, oncologist David Gorski wrote in Science-Based Medicine that the right-to-try law is harmful to society as it is popular with the public who do not understand how the FDA works, Gorski calls this "placebo legislation. They make lawmakers feel good, but they do nothing concrete to help actual patients." Gorski states that right-to-try laws enable "cancer quack" like the Burzynski Clinic to operate for years. "It's also important to remember that the real purpose of right-to-try laws is not to help patients, but to neuter the FDA's ability to regulate certain drugs, consistent with the source of this legislation." Gorski further states that these laws "rest on a fantasy... of false hope ... that is rooted in libertarian politics ... that claims that deregulation is the cure for everything."[33]
Future
In 2016, Houston oncologist Dr. Ebrahim Delpassand testified to a US Senate committee that he treated 78 patients for neuroendocrine cancer with LU-177 octreotate under the Texas Right to Try law, after the FDA refused permission to include those patients in the clinical trial that he was running.[34][35] However, the drug's manufacturer, Advanced Accelerator Applications, has made this drug available through an expanded access program for patients with neuroendocrine tumors, so it is disputed whether this is a substantiated case of a right to try law being used to gain patients access to an investigational product.[36] The laws remain wildly popular, and state legislators continue to introduce bills.
References
- 1 2 Turkowitz, Julie (January 10, 2015). "Patients Seek 'Right to Try' New Drugs". New York Times. Retrieved August 14, 2015.
- 1 2 "In Your State Right to Try - Movement for Terminal Patients". Goldwater Institute. Retrieved March 13, 2018.
Those states not showing adoption (by image inspection) - Alaska, Hawaii, New Mexico, Nebraska, Kansas, Wisconsin, New York, Vermont, Massachusetts, New Jersey, Delaware
- ↑ "'Right to Try' Law Gives Terminal Patients Access to Drugs Not Approved by FDA". PBS NewsHour (Transcript of television program). NewsHour Productions LLC. June 21, 2014. Retrieved March 13, 2018.
This May, Colorado's Democratic governor signed the nation's first 'right to try' bill.
- ↑ "Oregon 24th state to enact 'Right to Try' law" (Press release). Goldwater Institute. August 13, 2015. Archived from the original on August 16, 2015 – via KTVZ.com.
- ↑ Tenth Amendment Center Blog | Signed by the Governor: South Carolina Right to Try Act Rejects Some FDA Restrictions on Terminal Patients
- ↑ California Becomes 32nd State to Pass "Right to Try" Law for Terminally Ill - Hit & Run : Reason.com
- ↑ "S.204 - Trickett Wendler, Frank Mongiello, Jordan McLinn, and Matthew Bellina Right to Try Act of 2017". 115th U.S. Congress, 1st session.
- ↑ "Senate Passes 'Right to Try' Bill". Regulatory Affairs Professional Society.
- ↑ "Johnson Agrees To Drop Hold On FDARA In Exchange For Vote On Revised Right-To-Try Bill". Inside Health Policy.
- ↑ "H.R.878". 115th U.S. Congress, 1st session. Retrieved March 17, 2017.
- ↑ Karlin-Smith, Sarah (March 21, 2018). "House passes right-to-try bill on second try". Politico. Retrieved May 11, 2018.
- ↑ Tomoski, Miroslav (2018-05-24). "Trump To Sign Bill Which Could Make Cannabis Federally Legal For The Terminally Ill". Herb.co. Retrieved 2018-05-25.
- ↑ Hellmann, Jessie (2018-05-30). "Trump signs 'right to try' drug bill". TheHill. Retrieved 2018-05-30.
- ↑ "Goldwater Institute Right to Try Model Legislation" (PDF). Archived from the original (PDF) on January 15, 2016. Retrieved February 19, 2016.
- ↑ Monir, M (February 19, 2015). "States Move to Give Terminally Ill 'Right-to-Try' Drugs". USA Today. Retrieved August 14, 2015.
- ↑ Harada, T (May 9, 2014). "Afflicted Have the Right to Try". Atlanta Journal-Constitution. Retrieved February 19, 2016.
- ↑ Andriote, J-M. "Who Decides? 'Right-to-Try' Law's Unacknowledged, Deep Roots in AIDS Activism" (May 22, 2014). Huffington Post. Retrieved February 19, 2016.
- ↑ Cohen-Kurzrock, J (May 24, 2016). "Health policy: The right to try is embodied in the right to die". Nature Reviews. Retrieved November 20, 2016.
- ↑ Dennis, B, Eunjung Cha, A (May 15, 2014). "'Right to Try' Laws Spur Debate Over Dying Patients' Access to Experimental Drugs". Washington Post. Retrieved August 14, 2015.
- ↑ Darrow, J; et al. (January 15, 2015). "Practical, Legal, and Ethical Issues in Expanded Access to Investigational Drugs". New England Journal of Medicine.
- ↑ Munz, M (May 20, 2015). "Missouri's 'Right to Try' Law No Guarantee Patient Will Get Experimental Drugs". St. Louis Post-Dispatch. Retrieved February 19, 2016.
- ↑ Bateman-House, A, Caplan, A (August 13, 2015). "All Hat, No Cattle—The False Hope of Right to Try Laws". Harvard Health Policy Review. Retrieved February 19, 2016.
- ↑ Bateman-House, A; et al. (September 17, 2015). "Right-to-Try Laws: Hope, Hype, and Unintended Consequences". Annals of Internal Medicine. Retrieved February 19, 2016.
- ↑ Kearns, L, Caplan, A (April 29, 2015). "Right-to-Try Legislation Punishing". Albany Times Union. Retrieved February 19, 2016.
- ↑ Leonard, K (November 18, 2014). "Seeking the Right to Try". U.S. News & World Report. Retrieved August 14, 2015.
- ↑ Gorski, D (July 21, 2014). "The False Hope of 'Right to Try' Metastasizes to Michigan". Science-Based Medicine. Retrieved February 19, 2016.
- ↑ Temin, P. Taking Your Medicine: Drug Regulation in the United States. Cambridge: Harvard University Press. pp. 1–2.
- ↑ Silverman, E (October 12, 2015). "'Right to Try' Laws Wrong to Skirt FDA". Boston Globe. Retrieved February 19, 2016.
- ↑ Yang, T; et al. (July 20, 2015). "'Right to Try' Legislation: Progress or Peril?". Journal of Clinical Oncology. Retrieved February 19, 2016.
- ↑ Adriance, S (December 4, 2014). "Fighting for the 'Right to Try' Unapproved Drugs: Law as Persuasion". Yale Law Journal. Retrieved February 19, 2016.
- ↑ Farber, D; et al. (May 22, 2015). "How State Right-to-Try Laws Create False Expectations". Health Affairs Blog. Retrieved February 19, 2016.
- ↑ Zettler, P, Greely, H (December 1, 2014). "The Strange Allure of State 'Right-to-Try' Laws". JAMA Internal Medicine. Retrieved February 19, 2016.
- ↑ Gorski, David. "The cruel sham that is right-to-try raises its ugly head at the federal level again". Science-Based Medicine. Archived from the original on 31 May 2018. Retrieved 31 May 2018.
- ↑ "Statement of Sen. Johnson of Wisconsin". The Congressional Record. 2016-09-28. Retrieved 2017-02-10.
- ↑ Kevin McCormack (December 19, 2016). "'Right To Try' laws called 'Right To Beg' by Stem Cell Advocates". The Stem Cellar, The Official Blog of CIRM, California's Stem Cell Agency. Retrieved 2017-02-10.
- ↑ "Expanded Access Protocol for Therapeutic Use of 177Lu-DOTA0-Tyr3-Octreotate in Patients With Inoperable, Somatostatin Receptor Positive, Midgut Carcinoid Tumors, Progressive Under Somatostatin Analogue Therapy". ClinicalTrials.gov. 2017-02-17. Retrieved 2017-03-17.
External links
- Hearing, Examining Patient Access to Investigational Drugs. The Health Subcommittee Of The Committee On Energy And Commerce. U.S. House Of Representatives. October 3, 2017