Pyoluteorin
 | |
| Identifiers | |
|---|---|
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C11H7Cl2NO3 |
| Molar mass | 272.08 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Pyoluteorin is a natural antibiotic that is biosynthesized from a hybrid nonribosomal peptide synthetase (NRPS) and polyketide synthase (PKS) pathway.[1] Pyoluteorin was first isolated in the 1950s from Pseudomonas aeruginosa strains T359 and IFO 3455[2] and was found to be toxic against oomycetes, bacteria, fungi, and against certain plants.[3] Pyoluteorin is most notable for its toxicity against the oomycete Pythium ultimum,[4] which is a plant pathogen that causes a global loss in agriculture. Currently, pyoluteorin derivatives are being studied as an Mcl-1 antagonist in order to target cancers that have elevated Mcl-1 levels.[5]
Biosynthesis
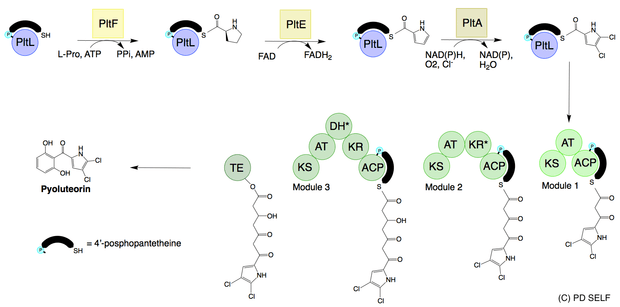
Pyoluteorin is synthesized from an NRPS/PKS hybrid pathway. The resorcinol ring is derived from a type I PKS[6][7] while the dichloropyrrole moiety is derived from a type II NRPS.[8] Pyoluteorin biosynthesis begins with the activation of L-proline to prolyl-AMP by the adenylation domain PltF. With prolyl-AMP still in the active site, the active form of the peptidyl carrier protein PltL binds to PltF. Then PltF catalyzes the aminoacylation of PltL by attaching L-proline to the thiol of the 4’phosphopantetheine arm of PltL.[9] Next, the dehydrogenase PltE desaturates the prolyl moiety on PltL to create pyrrolyl-PltL. The halogenation domain PltA then dichlorinates the pyrrole moiety first at position 5 and then at position 4 in a FADH2 dependent manner.[10] The dichloropyrroyl residue is then transferred to the type I PKS PltB and PltC, however, the mechanism of transfer is unknown. The addition of 3 malonyl-CoA monomers, cyclization, and release by the thioesterase PltG gives pyoluteorin.
References
- ↑ Gross, Harald (October 1, 2009). "Genomics of secondary metabolite production by Pseudomonas spp". Natural Product Reports. 26 (11): 1408–1446. doi:10.1039/b817075b. PMID 19844639.
- ↑ Takeda, Rokuro (1958). "Structure of a new antibiotic, pyoluteorin". Journal of the American Chemical Society. 80 (17): 4749–4750. doi:10.1021/ja01550a093.
- ↑ Maurhofer, M. (September 10, 1991). "Influence of Enhanced Antibiotic Production in Pseudomonas fluorescens Strain CHA0 on its Disease Suppressive Capacity". Phytopathology. 82 (2): 190–195. doi:10.1094/Phyto-82-190.
- ↑ Howell, C.R. (January 16, 1980). "Supression of Pythium ultimum-induced damping-off of cotton seedlings by pseudomonas fluorescens and its antibiotic, pyoluteorin". Phytopathology. 70: 712–715.
- ↑ Doi, Kenichiro (October 2014). "Characterization of pyoluteorin derivatives as Mcl-1 antagonists". American Association for Cancer Research. 74 (19): 1805. doi:10.1158/1538-7445.AM2014-1805.
- ↑ Cuppels, D.A. (January 15, 1986). "Biosynthesis of Pyoluteorin: A Mixed Polyketide-Tricarboxylic Acid Cycle Origin Demonstrated by [l,2-13C2]Acetate Incorporation". Naturforsch. 41c: 532–536.
- ↑ Nowak-Thompsonab, Brian (December 19, 1997). "Identification and sequence analysis of the genes encoding a polyketide synthase required for pyoluteorin biosynthesis in Pseudomonas fluorescens Pf-5". Gene. 204 (1–2): 17–24. doi:10.1016/S0378-1119(97)00501-5. PMID 9434161.
- ↑ Nowak-Thompson, Brian (April 10, 1999). "Characterization of the pyoluteorin biosynthetic gene cluster of Pseudomonas fluorescens Pf-5". Journal of Bacteriology. 181 (7): 2166–2174.
- ↑ Thomas, MG (October 4, 2001). "Conversion of L-Proline to Pyrrolyl-2-Carboxyl-S-PCP during Undecylprodigiosin and Pyoluteorin Biosynthesis". Cell Press. 9 (2): 171–184. doi:10.1016/S1074-5521(02)00100-X.
- ↑ Dorrestein, Pieter (September 27, 2005). "Dichlorination of a pyrrolyl-S-carrier protein by FADH2-dependent halogenase PltA during pyoluteorin biosynthesis". PNAS. 103 (39): 13843–13848. doi:10.1073/pnas.0506964102. PMC 1236592. PMID 16162666.