Pudovik reaction
In organophosphorus chemistry, the Pudovik reaction is a method for preparing α-aminomethylphosphonates. Under basic conditions, the phosphorus–hydrogen bond of a dialkylphosphite, (RO)2P(O)H, adds across the carbon–nitrogen double bond of an imine.[1] The reaction is closely related to the three-component Kabachnik–Fields reaction, where an amine, phosphite, and an organic carbonyl compound are condensed,[2] which was reported independently by Martin Kabachnik[3] and Ellis Fields[4] in 1952. In the Pudovik reaction, a generic imine, RCH=NR', would react with a phosphorous reagent like diethylphosphite as follows:[5]
- RCH=NR' + (EtO)2P(O)H → (EtO)2P(O)CHR-NHR'
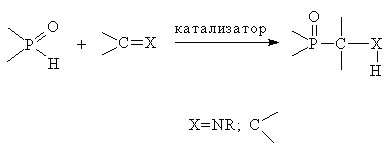
References
- ↑ Engel, Robert (2004). "Phosphorus Addition at sp2 Carbon". Organic Reactions. 36 (2): 175–248. doi:10.1002/0471264180.or036.02.
- ↑ Keglevich, György; Bálint, Erika (2012). "The Kabachnik-Fields reaction: Mechanism and synthetic use". Molecules. 17 (11): 12821–12835. doi:10.3390/molecules171112821.
- ↑ Kabachnik, Martin I.; Medved, T. Ya. (1952). "Новый метод синтеза сс-аминофосфиновых кислот" [A new method for the synthesis of α-amino phosphoric acids]. Doklady Akademii Nauk SSSR (in Russian). 83: 689ff.
- ↑ Fields, Ellis K. (1952). "The synthesis of esters of substituted amino phosphonic acids". Journal of the American Chemical Society. 74 (6): 1528–1531. doi:10.1021/ja01126a054.
- ↑ Ali, Tarik E.; Abdel-Kariem, Somaia M. (2015). "Methods for the synthesis of α-heterocyclic/heteroaryl-α-aminophosphonic acids and their esters". Arkivoc: Reviews and Accounts. 2015 (6): 246–287. doi:10.3998/ark.5550190.p009.112.
This article is issued from
Wikipedia.
The text is licensed under Creative Commons - Attribution - Sharealike.
Additional terms may apply for the media files.