Kabachnik–Fields reaction
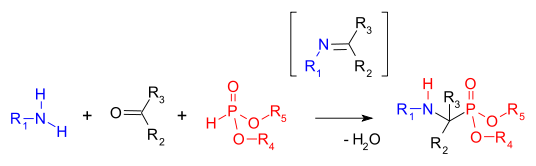
In organophosphorus chemistry, the Kabachnik–Fields reaction is a three-component organic reaction forming α-aminomethylphosphonates from an amine, a carbonyl compound, and a dialkyl phosphonate, (RO)2P(O)H (that are also called dialkylphosphites).[1] Aminophosphonates are synthetic targets of some importance as phosphorus analogues of α-amino acids (a bioisostere). This multicomponent reaction was independently discovered by Martin Izrailevich Kabachnik[2] and Ellis K. Fields[3] in 1952. The reaction is very similar to the two-component Pudovik reaction, which involves condensation of the phosphite and a preformed imine.
The first step in this reaction is the formation of an imine followed by an addition of the phosphonate P-H bond across the C=N double bond.[4] A related reaction is the Mannich reaction.
The reaction is accelerated with a combination of dehydrating reagent and Lewis acid. The carbonyl component in the reaction is usually an aldehyde and sometimes a ketone.
References
- ↑ Keglevich, Gyorgy; Balint, Erika (2012). "The Kabachnik-Fields reaction: mechanism and synthetic use". Molecules. 17: 12821–12835. doi:10.3390/molecules171112821.
- ↑ Kabachnik, Martin I.; Medved, T. Ya. (1952). "Новый метод синтеза сс-аминофосфиновых кислот" [A new method for the synthesis of α-amino phosphoric acids]. Doklady Akademii Nauk SSSR (in Russian). 83: 689ff.
- ↑ Fields, Ellis K. (1952). "The synthesis of esters of substituted amino phosphonic acids". Journal of the American Chemical Society. 74 (6): 1528–1531. doi:10.1021/ja01126a054.
- ↑ Zefirov, Nikolay S.; Elena D. Matveeva (2008-01-18). "Catalytic Kabachnik-Fields reaction: New horizons for old reaction" (PDF). ARKIVOC. 2008 (i): 1–17. doi:10.3998/ark.5550190.0009.101. Retrieved 2009-12-08.