Position-specific isotope analysis
Position-specific isotope analysis (PSIA), also called site-specific isotope analysis, is a branch of isotope analysis aimed at determining the isotopic composition of a particular atom position in a molecule. It can be described as the determination of the relative concentration of isotopomers at natural abundance. The first evidence for a deviation from a stochastic distribution was found in 1961 on amino acids whose carboxyl groups were 13C-enriched compared to the rest of the molecule.[1] Since then, several studies have shown that PSIA can give insights into the origin of a given molecule: biosynthetic pathway, mechanism and temperature of formation.
Principle
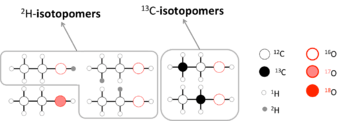
Following a pure stochastic (or "random") distribution, isotopomers of a given molecules should be present in the same amount. For instance, 13CH3-12CH2OH and 12CH3-13CH2OH are expected to have the same concentration. Yet, due to differences in the rate and/or equilibrium constant of a given reaction, an isotopomer can react faster than its homologue, creating a deviation from a stochastic distribution.
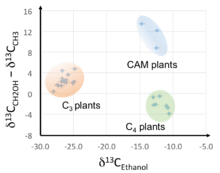
For example, C3 plants are known to assimilate preferentially 12CO2 compared with C4 plants, leading to different 13C isotope composition of the biomass (Figure 2, X-axis). Recent results on ethanol show that the relative abundances of isotopomers are not equal, and that the amplitude and sign of the deviation is dependent on the plant. These variations are thought to arise from biosynthetic isotope effects and different metabolic routes, especially those associated with the biosynthesis of sugars which are converted into ethanol during fermentation.[2][3]
Techniques
The difficulty of position-specific isotope analyses relies on the fact that isotopomers have the same mass and are thus difficult to separate using conventional isotopic measurements. Several methods can be used to overcome these difficulties.
Chemical degradation: the molecule is degraded chemically into smaller fragments. Each of the fragment is separated and its isotopic composition analysed using a conventional isotope ratio mass spectrometer.[4][5]
Pyrolysis: the sample is introduced into a pyrolysis furnace (which temperature is usually held between 600 °C and 1000 °C). Thermal cracking leads to the formation of fragments that are subsequently analyzed by IRMS.[6]
High-resolution mass spectrometry: the molecule is fragmented by electron impact directly in the ion source of the spectrometer.
Nuclear Magnetic Resonance (NMR): each non-equivalent isotopomer of the molecule leads to a peak at a specific chemical shift. Their relative concentration is determined through the area of each peak. (Link: Isotopic analysis by nuclear magnetic resonance).[7]
Density functional theory calculations: equilibrium isotope effects at the position-specific levels can be computed through DFT calculations for different temperatures.[8]
References
- ↑ Abelson PH, Hoering TC. CARBON ISOTOPE FRACTIONATION IN FORMATION OF AMINO ACIDS BY PHOTOSYNTHETIC ORGANISMS. Proceedings of the National Academy of Sciences of the United States of America. 1961;47(5):623-632.
- ↑ Schmidt, Hanns-Ludwig (2003-11-20). "Fundamentals and systematics of the non-statistical distributions of isotopes in natural compounds". Naturwissenschaften. 90 (12): 537–552. Bibcode:2003NW.....90..537S. doi:10.1007/s00114-003-0485-5. ISSN 0028-1042.
- ↑ Gilbert, Alexis; Robins, Richard J.; Remaud, Gérald S.; Tcherkez, Guillaume G. B. (2012-10-30). "Intramolecular 13C pattern in hexoses from autotrophic and heterotrophic C3 plant tissues". Proceedings of the National Academy of Sciences. 109 (44): 18204–18209. Bibcode:2012PNAS..10918204G. doi:10.1073/pnas.1211149109. ISSN 0027-8424. PMC 3497804. PMID 23074255.
- ↑ Monson, K. D.; Hayes, J. M. (1980-12-10). "Biosynthetic control of the natural abundance of carbon 13 at specific positions within fatty acids in Escherichia coli. Evidence regarding the coupling of fatty acid and phospholipid synthesis". Journal of Biological Chemistry. 255 (23): 11435–11441. ISSN 0021-9258. PMID 7002923.
- ↑ Gelwicks, Jeffrey T.; Hayes, J. M. (1990-03-01). "Carbon-isotopic analysis of dissolved acetate". Analytical Chemistry. 62 (5): 535–539. doi:10.1021/ac00204a021. ISSN 0003-2700.
- ↑ Corso, Thomas N.; Brenna, J. Thomas (1997-02-18). "High-precision position-specific isotope analysis". Proceedings of the National Academy of Sciences. 94 (4): 1049–1053. Bibcode:1997PNAS...94.1049C. doi:10.1073/pnas.94.4.1049. ISSN 0027-8424. PMC 19741. PMID 11038597.
- ↑ Tenailleau, Eve; Lancelin, Pierre; Robins, Richard J.; Akoka, Serge (2004-07-01). "NMR Approach to the Quantification of Nonstatistical 13C Distribution in Natural Products: Vanillin". Analytical Chemistry. 76 (13): 3818–3825. doi:10.1021/ac0496998. ISSN 0003-2700.
- ↑ Piasecki, Alison; Sessions, Alex; Peterson, Brian; Eiler, John (2016-10-01). "Prediction of equilibrium distributions of isotopologues for methane, ethane and propane using density functional theory". Geochimica et Cosmochimica Acta. 190: 1–12. Bibcode:2016GeCoA.190....1P. doi:10.1016/j.gca.2016.06.003.