Plant defensin
| Plant defensins | |||||||||
|---|---|---|---|---|---|---|---|---|---|
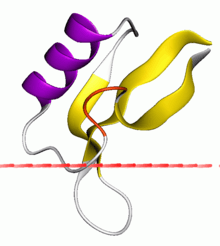 Antifungal protein 1 | |||||||||
| Identifiers | |||||||||
| Symbol | Gamma-thionin | ||||||||
| Pfam | PF00304 | ||||||||
| Pfam clan | CL0054 | ||||||||
| InterPro | IPR008176 | ||||||||
| PROSITE | PDOC00725 | ||||||||
| SCOP | 1gps | ||||||||
| SUPERFAMILY | 1gps | ||||||||
| OPM superfamily | 58 | ||||||||
| OPM protein | 1jkz | ||||||||
| CDD | cd00107 | ||||||||
| |||||||||
Plant defensins (Formerly gamma-thionins) are a family of small, cysteine-rich proteins found in plants that serve to defend them against parasites.
The following plant proteins belong to this family:
- The flower-specific Nicotiana alata defensin (NaD1)
- Gamma-thionins from Triticum aestivum (Wheat) endosperm (gamma-purothionins) and gamma-hordothionins from Hordeum vulgare(Barley) are toxic to animal cells and inhibit protein synthesis in cell free systems.[1]
- A flower-specific thionin (FST) from Nicotiana tabacum (Common Tobacco).[2]
- Antifungal proteins (AFP) from the seeds of Brassicaceae species such as radish, mustard, turnip and Arabidopsis thaliana (Thale Cress).[3]
- Inhibitors of insect alpha-amylases from sorghum.[4]
- Probable protease inhibitor P322 from Solanum tuberosum (Potato).
- A germination-related protein from Vigna unguiculata (Cowpea).[5]
- Anther-specific protein SF18 from sunflower. SF18 is a protein that contains a gamma-thionin domain at its N-terminus and a proline-rich C-terminal domain.
- Glycine max (Soybean) sulfur-rich protein SE60.[6]
- Vicia faba (Broad bean) antibacterial peptides fabatin-1 and -2.
In their mature form, these proteins generally consist of about 45 to 50 amino-acid residues. As shown in the following schematic representation, these peptides contain eight conserved cysteines involved in disulfide bonds.
+-------------------------------------------+
| +-------------------+ |
| | | |
xxCxxxxxxxxxxCxxxxxCxxxCxxxxxxxxxCxxxxxxCxCxxxC
| | | |
+---|----------------+ |
+------------------+
'C': conserved cysteine involved in a disulfide bond.
The folded structure of Gamma-purothionin is characterised by a well-defined 3-stranded anti-parallel beta-sheet and a short alpha-helix.[1] Three disulfide bridges are located in the hydrophobic core between the helix and sheet, forming a cysteine-stabilised alpha-helical motif. This structure differs from that of the plant alpha- and beta-thionins, but is analogous to scorpion toxins and insect defensins.
Databases
A database for antimicrobial peptides, including defensins is available: PhytAMP (http://phytamp.hammamilab.org).[7]
References
- 1 2 Bruix M, Jime nez MA, Santoro J, Gonzalez C, Colilla FJ, Mendez E, Rico M (1993). "Solution structure of gamma 1-H and gamma 1-P thionins from barley and wheat endosperm determined by 1H-NMR: a structural motif common to toxic arthropod proteins". Biochemistry. 32 (2): 715–724. doi:10.1021/bi00053a041. PMID 8380707.
- ↑ Gu Q, Kawata EE, Cheung AY, Morse MJ, Wu HM (1992). "A flower-specific cDNA encoding a novel thionin in tobacco". Mol. Gen. Genet. 234 (1): 89–96. doi:10.1007/BF00272349. PMID 1495489.
- ↑ Osborn RW, Torrekens S, Vanderleyden J, Broekaert WF, Cammue BP, Terras FR, Van Leuven F (1993). "A new family of basic cysteine-rich plant antifungal proteins from Brassicaceae species". FEBS Lett. 316 (3): 233–240. doi:10.1016/0014-5793(93)81299-F. PMID 8422949.
- ↑ Richardson M, Bloch Jr C (1991). "A new family of small (5 kDa) protein inhibitors of insect alpha-amylases from seeds or sorghum (Sorghum bicolar (L) Moench) have sequence homologies with wheat gamma-purothionins". FEBS Lett. 279 (1): 101–104. doi:10.1016/0014-5793(91)80261-Z. PMID 1995329.
- ↑ Ishibashi N, Yamauchi D, Minamikawa T (1990). "Stored mRNA in cotyledons of Vigna unguiculata seeds: nucleotide sequence of cloned cDNA for a stored mRNA and induction of its synthesis by precocious germination". Plant Mol. Biol. 15 (1): 59–64. doi:10.1007/BF00017724. PMID 2103443.
- ↑ Choi Y, Choi YD, Lee JS (1993). "Nucleot ide sequence of a cDNA encoding a low molecular weight sulfur-rich protein in soybean seeds". Plant Physiol. 101 (2): 699–700. doi:10.1104/pp.101.2.699. PMC 160625. PMID 8278516.
- ↑ Hammami R, Ben Hamida J, Vergoten G, Fliss I (2008). "PhytAMP: a database dadicated to plant antimicrobial peptides". Nucleic Acids Research. 37 (Database issue): D963. doi:10.1093/nar/gkn655. PMC 2686510. PMID 18836196.