Perfluoroalkoxy alkane
| PFA | |
|---|---|
 | |
| Density[1] | 2150 kg/m3 |
| Flexural modulus(E) | 586 MPa |
| Tensile strength(t) | 24 MPa |
| Elongation at break | 300% |
| Folding endurance | No break |
| Notch test | |
| Melting point | 315 °C |
| Maximum operating | |
| temperature | 260 °C |
| Water absorption (ASTM) | <0.03 % after 24 hours |
| Dielectric constant (Dk) | |
| at 1MHz | 2.1 |
| Dissipation factor | |
| at 1MHz | 0.0001 |
| Arc resistance | < 180 seconds |
| Resistivity at 50% R. H. | > 1016 Ω m |

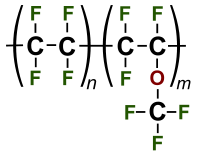
Perfluoroalkoxy alkanes (PFA) are fluoropolymers. They are copolymers of tetrafluoroethylene (C2F4) and perfluoroethers (C2F3ORf, where Rf is a perfluorinated group such as trifluoromethyl (CF3)). In terms of their properties, these polymers are similar to polytetrafluoroethylene (PTFE). The big difference is that the alkoxy substituents allow the polymer to be melt-processed. On a molecular level, PFA has a smaller chain length and higher chain entanglement than other fluoropolymers. It also contains an oxygen atom at the branches. This results in a material that is more translucent and has improved flow, creep resistance, and thermal stability close to or exceeding PTFE.[2] Similarly advantaged processing properties are found in fluorinated ethylene propylene (FEP), the copolymer of tetrafluoroethylene and hexafluoropropylene.[3]
Applications
PFA is commonly used as material for piping and fittings for aggressive chemicals, as well as corrosion-resistant lining of vessels in the chemical-processing industry. Typical applications are in the construction of gas scrubbers, reactors, containment vessels, and piping.[4] In coal-fired power plants, it is used as lining for heat exchangers. By channeling crude gas through the PFA-lined apparatus the gas stream can be cooled beyond its condensation temperature without damaging the heat exchanger. Its use contributes to increasing the efficiency of the whole plant.[5]
References
- ↑ "PTFE, FEP, and PFA Specifications". Boedeker Corp. 2007. Retrieved 2007-12-22.
- ↑ "PFA Properties". Fluorotherm Polymers. 2010. Archived from the original on 2014-02-22. Retrieved 2014-02-12.
- ↑ Günter Siegemund, Werner Schwertfeger, Andrew Feiring, Bruce Smart, Fred Behr, Herward Vogel, Blaine McKusick "Fluorine Compounds, Organic" Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH, Weinheim, 2002. doi:10.1002/14356007.a11_349.
- ↑ Dietrich Braun, Kunststofftechnik für Einsteiger, Hanser, München, 2003.
- ↑ H. Saechtling: Kunststoff Taschenbuch, Hanser Verlag, Wien 1995, ISBN 3-446-17855-4.