Pathogenomics
Pathogen infections are among the leading causes of infirmity and mortality among humans and other animals in the world.[1] Until recently, it has been difficult to compile information to understand the generation of pathogen virulence factors as well as pathogen behaviour in a host environment. The study of pathogenomics attempts to utilize genomic and metagenomics data gathered from high through-put technologies (e.g. sequencing or DNA microarrays), to understand microbe diversity and interaction as well as host-microbe interactions involved in disease states. The bulk of pathogenomics research concerns itself with pathogens that affect human health; however, studies also exist for plant and animal infecting microbes.
History
In the early investigation of microbial genomics, it was difficult and costly to obtain sequence information for any pathogen. In 1995, the first pathogen genome, that of Haemophilus influenza, was sequenced by traditional Sanger methods. Sanger methods, however, were slow and costly.[2] The emergence of second-generation high-throughput sequencing technologies has allowed for microbial sequence information to be obtained much more quickly and at a considerably lower cost.[3][4] Largely thanks to second-generation sequencing methods, tens of thousands of pathogen genomes have been sequenced since 1995. The emergence of second-generation high-throughput sequencing technologies has allowed for microbial sequence information to be obtained much more quickly and at a considerably lower cost.[5] This influx of information is also due to the capacity of sequencing platforms to generate the sequences of many organisms in parallel.[6]
With the sequences of many organisms available for analysis, scientists, through their investigations, began to challenge some of the earlier tenets of bacterial genome structure. Older paradigms of microbial genomics believed that only a few strains were sufficient to represent a specific bacterial species.[3][5][7]
It was thought that bacterial genomes, like eukaryotes, were relatively stable. In 2001, however, the sequences of Escherichia coli 0157:H7 was obtained in a study by Perna and her colleagues; the study showed that two members of the same bacterial species can differ as much as 30% in genomic content.[8] It became evident that sequencing multiple strains for a species, rather than a few selectively chosen ones, was necessary to understand the diversity in a microbial species gene pool. It was also increasingly important to understand how to account for these differences in genomic content across a species strains and how it may contribute to pathogenic behaviour or prevent the formation of pathogens.[5]
More recently, the sequenced genomic data have been catalogued in databases and made publicly available online (there also exist non-publicly available databases in the private sector). The availability and influx of this information presses upon those who conduct pathogenomics research to come up with a way of drawing meaningful conclusions from these data. In addition, the availability of such data on the Internet encourages global collaboration of labs.
Microbe analysis
Pathogens may be prokaryotic (archaea or bacteria), single-celled Eukarya or viruses. Prokaryotic genomes have typically been easier to sequence due to smaller genome size compared to Eukarya.[5] Due to this, there is a bias in reporting pathogenic bacterial behaviour. More recently there have been increased efforts to sequence Eukarya genomes and more will be underway in the future.[5] Regardless of this bias in reporting, many of the dynamic genomic events are similar across all the types of pathogen organisms.
Pathogenomics does not focus exclusively on understanding pathogen-host interactions. Insight of individual or cooperative pathogen behaviour provides knowledge into the development or inheritance of pathogen virulence factors.[3] Through a deeper understanding of the small subunits that cause infection, it may be able possible to develop novel therapeutics that are efficient and cost effective. [9]
Analyzing individual microbes
Dynamic genomes with high plasticity are necessary to allow pathogens, especially bacteria, to survive in changing environments.[5] With the assistance of high throughput sequencing methods and in silico technologies, it is possible to detect, compare and catalogue many of these dynamic genomic events. Particular interest is in understanding how genomic events lead to pathogen development and how these events may be interrupted to prevent it.
Causes of genomic diversity
Three forces act in shaping the pathogen genome: gene gain, gene loss, and genome rearrangement.[3] The knowledge and detection of these genomic dynamic events are necessary in the construction of useful therapeutic tools to combat pathogens.
Bacterial genome evolutionary dynamics
Gene loss and genome decay
Gene loss or genome decay occurs when a gene is no longer used by the microbe or when a microbe attempts to adapt to a new ecological niche.[10]
Sequencing efforts and microarray analysis have exposed a large number of pseudo genes in some bacterial pathogen species. Mycobacterium leprae for example has been found to contain nearly as many pseudogenes as function genes.[11] M. leprae is not the only microbe exhibiting such behaviour; in his article, Pallen [3] reports similar properties from Yersinia pestis (the plague pathogen) [12] and also Salmonella enterica.[13] The inactivation of genes is typically associated with a change in the lifestyle of an organism, which can involve adapting to a new niche. The presence of extensive pseudogenes is contrary to another orthodox belief that all the genes in a bacterial genome are functional for some purpose[3]
It is possible to detect the presence of pseudogenes and the marks of genome decay through whole-genome sequencing in combination with comparative genomics [3] Comparative genomics has helped to reveal that pathogens may favour losing genes in order to live in a host-associated niche and become endosymbionts. Sometimes the shedding of certain genes also renders a pathogen microbe harmless. The analysis of Listeria strains, for example, has shown that a reduced genome size has led to the generation of a non-pathogenic Listeria strain from a pathogenic precursor.[14]
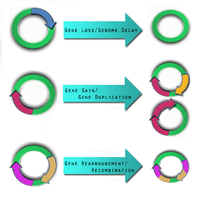
Gene gain and gene duplication
One of the key forces driving gene gain is thought to be horizontal (lateral) gene transfer (LGT).[15] It is of particular interest in microbial studies because these mobile genetic elements may introduce virulence factors into a new genome.[16] A comparative study conducted by Gill et al. in 2005 postulated that LGT may have been the cause for pathogen variations between Staphylococcus epidermidis and Staphylococcus aureus.[17] There still, however, remains scepticism about the frequency of LGT, its identification, and its impact.[18]
New and improved methodologies have been engaged, especially in the study of phylogenetics, to validate the presence and effect of LGT.[19]
Gene gain and gene duplication events are balanced by gene loss, such that despite their dynamic nature, the genome of a bacterial species remains approximately the same size.[20]
Genome rearrangement
Mobile genetic insertion sequences can play a role in genome rearrangement activities.[12] Pathogens that do not live in an isolated environment have been found to contain a large number of insertion sequence elements and various repetitive segments of DNA.[5] The combination of these two genetic elements is thought help mediate homologous recombination. There are pathogens, such as Burkholderia mallei,[21] and Burkholderia pseudomallei [22] which have been shown to exhibit genome-wide rearrangements due to insertion sequences and repetitive DNA segments.[5] At this time, no studies demonstrate genome-wide rearrangement events directly giving rise to pathogenic behaviour in a microbe. This does not mean it is not possible. Genome-wide rearrangements do, however, contribute to the plasticity of bacterial genome, which may prime the conditions for other factors to introduce, or lose, virulence factors.[5]
Single-nucleotide polymorphism
Single nucleotide polymorphisms (SNPs) are also a genomic variable that adds to the diversity of pathogen strains. Current efforts attempt to catalogue the various SNPs in pathogen strains.[1]
Analysis of genomic diversity
There is a need to analyze more than a single genome sequence of a pathogen species to understand pathogen mechanisms. Comparative genomics is a methodology that has gained more applicability with the recent increased amount of sequence information. There are several examples of successful comparative genomics studies, among them the analysis of Listeria.[14] and Escherichia coli.[8] The most important topic comparative genomics, in a pathogenomic context, attempts to address the difference between pathogenic and non-pathogenic microbes. This inquiry, though, proves to be very difficult to analyze, since a single bacterial species can have many strains and the genomic content of each of these strains can vary.[8]
Pan-genomes and core genomes
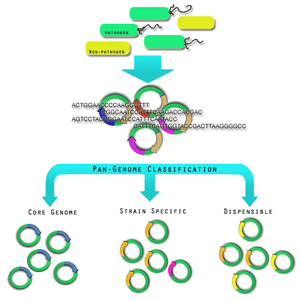
The diversity within pathogen genomes makes it difficult to identify the total number of genes that are associated within all strains of a pathogen species.[23] It has been thought that the total number of genes associated with a single pathogen species may be unlimited,[23] although some groups are attempting to derive a more empirical value.[24] For this reason it was necessary to introduce the concept of pan-genomes and core genomes.[25] Pan-genome and core genome literature also tends to have a bias towards reporting on prokaryotic pathogen organisms. Caution may need to be exercised when extending the definition of a pan-genome or a core-genome to the other pathogen organisms; this is because there is no formal evidence of the properties of these pan-genomes. Here, it will be assumed that the definitions may extend, since all pathogen organisms share in the same dynamic genomic events and rely upon variability within strains as a mechanism of survival and virulence.
A core genome is the set of genes found across all strains of a pathogen species.[23] A pan-genome is the entire gene pool for that pathogen species, and includes genes that are not shared by all strains.[23] Pan-genomes may be open or closed depending on whether comparative analysis of multiple strains reveals no new genes (closed) or many new genes (open) compared to the core genome for that pathogen species.[3] In the open pan-genome, genes may be further characterized as dispensable or strain specific. Dispensable genes are those found in more than one strain, but not in all strains, of a pathogen species.[25] Strain specific genes are those found only in one strain of a pathogen species.[25] The differences in pan-genomes are reflections of the life style of the organism. For example, Streptococcus agalactiae, which exists in diverse biological niches, has a broader pan-genome when compared with the more environmentally isolated Bacillus anthracis.[5] Comparative genomics approaches are also being used to understand more about the pan-genome.[26]
Mobile genetic elements that encode virulence factors
Three genetic elements of human-affecting pathogens contribute to the transfer of virulence factors: plasmids, pathogenicity island, and prophages.[3] Pathogencity islands and their detection are the focous of several bioinformatics efforts involved in pathogenomics.[27][28]
Analyzing microbe-microbe interactions

Microbe-host interactions tend to overshadow the consideration of microbe-microbe interactions. Microbe-microbe interactions though can lead to chronic states of infirmity that are difficult to understand and treat.[1]
Biofilms
Biofilms are an example of microbe-microbe interactions and are thought to be associated with up to 80% of human infections.[29] Recently it has been shown that there are specific genes and cell surface proteins involved in the formation of biofilm.[30] These genes and also surface proteins may be characterized through in silico methods to form an expression profile of biofilm-interacting bacteria.[1] This expression profile may be used in subsequent analysis of other microbes to predict biofilm microbe behaviour, or to understand how to dismantle biofilm formation.[1]
Host microbe analysis
A microbe may be influenced by hosts to either adapt to its new environment or learn to evade it. An insight into these behaviours will provide beneficial insight for potential therapeutics. The most detailed outline of host-microbe interaction initiatives is outlined by the Pathogenomics European Research Agenda.[1] Its report emphasises the following features:

- Microarray analysis of host and microbe gene expression during infection. This is important for identifying the expression of virulence factors that allow a pathogen to survive a host's defence mechanism.[1] Pathogens tend to undergo an assortment of changed in order to subvert and hosts immune system, in some case favouring a hyper variable genome state.[31] The genomic expression studies will be complimented with protein-protein interaction networks studies.[1]
- Using RNA interference (RNAi) to identify host cell functions in response to infections. Infection depends on the balance between the characteristics of the host cell and the pathogen cell. In some cases, there can be an overactive host response to infection, such as in meningitis, which can overwhelm the host's body.[1] Using RNA, it will be possible to more clearly identify how a host cell defends itself during times of acute or chronic infection.[32] This has also been applied successfully is Drosophila.[32]
- Not all microbe interactions in host environment are malicious. Commensal flora, which exists in various environments in animals and humans may actually help combating microbial infections.[1] The human flora, such as the gut for example, is home to a myriad of microbes.[33]
The diverse community within the gut has been heralded to be vital for human health. There are a number of projects under way to better understand the ecosystems of the gut.[34] The sequence of commensal Escherichia coli strain SE11, for example, has already been determined from the faecal matter of a healthy human and promises to be the first of many studies.[35] Through genomic analysis and also subsequent protein analysis, a better understanding of the beneficial properties of commensal flora will be investigated in hopes of understanding how to build a better therapeutic.[36]
Eco-evo perspective
The "eco-evo" perspective on pathogen-host interactions emphasizes the influences ecology and the environment on pathogen evolution.[3] The dynamic genomic factors such as gene loss, gene gain and genome rearrangement are all strongly influenced by changes in the ecological niche that a particular microbial strain resides in. Microbes may switch from being pathogenic and non-pathogenic due to changing environments.[14] Studies of the plague, Yersinia pestis, are prominent demonstration of how over time a microbe, in this case Yersinia pestis, evolves from a gastrointestinal pathogen to a very highly pathogenic microbe through dynamic genomic events.[37] These flips between being pathogenic and non-pathogenic status and how they occurred with respect to ecological or environmental changes are important in novel therapeutic development to combat microbial infections.[3]
Applications
Many of the future challenges of pathogenomics begin with handling and making sense of the large influx of data that now is available to the research community. Mining the data for useful information proves to be applicable to many facets of epidemiology. bioinformatics approaches are providing much of the power for rapidly mining, organising, analyzing, visualizing and annotating the data catalogued in databases.
Note that pathogenomics research could shed light for extensions of pathogens, or non-pathogens, that are not related to human, plant or animal health; using microbes for bioremediation is one example. There is some but very little dialogue, however, concerning these extensions to pathogens and their relation to pathogenomics. It would be more suitable to categorize pathogen/non-pathogen applications that are unrelated to infection under the more general category of microbe genomics. Some general reviews speak extensively both about pathogen related and non-related applications in the same article.[5]
In the advent of new technology, it is easy to forget some of the basic things that prevent pathogen infections from starting and spreading. While there exist more deadly and difficult-to-handle pathogen infections, there also exist less dangerous ones. Historically, human health greatly improved with more emphasis on healthy life styles including better hygiene practices and access to clean recourses of water and nutrition.
Reverse vaccinology
The variability of genomes can make the developed of a vaccine very difficult, and antigen variation cannot match pathogen variation. Reverse vaccinology is a novel approach that may develop vaccines to combat pathogens more effectively.[38] Reverse Vaccinology has already been successfully applied to Neisseria meningitides, Streptococcus pneumoniae and Chlamydia spp.[5] Reverse Vaccinology applies not only to strain specific vaccines, but also the development of pan-genome vaccines.[39] Lastly, comparative vaccinology attempts to compare the differences between pathogen and non-pathogen variants of a microbe to filter genes that are unique to the pathogen version.[38] There are several vaccines developed through reverse vaccinology that are currently in clinical trials.[39]
Countering bioterrorism
In 2005 the sequence of the 1918 Spanish influenza was completed. Accompanied with phylogenetic analysis, it was possible to supply a detailed account of the virus' evolution and behaviour, in particular its adaptation to humans.[40] Following the sequencing of the Spanish influenza, the pathogen was also reconstructed. When inserted into mice, the pathogen proved to be incredibly deadly.[41][42] The 2001 anthrax attacks shed light on the possibility of bioterrorism as being more of a real than imagined threat. Bioterrorism was anticipated in the Iraq war, with soldiers being inoculated for a smallpox attack.[43] Using technologies and insight gained from reconstruction of the Spanish influenza, it may be possible to prevent future deadly planted outbreaks of disease. There is a strong ethical concern however, as to whether the resurrection of old viruses is necessary and whether it is actually more harm than good.[42][44]
References
- 1 2 3 4 5 6 7 8 9 10 11 Demuth, A.; Aharonowitz, Y.; Bachmann, T. T.; Blum-Oehler, G.; Buchrieser, C.; Covacci, A.; Dobrindt, U.; Emödy, L.; Van Der Ende, A.; Ewbank, J.; Fernández, L. Á.; Frosch, M.; Portillo, F. G. A. D.; Gilmore, M. S.; Glaser, P.; Goebel, W.; Hasnain, S. E.; Heesemann, J. R.; Islam, K.; Korhonen, T.; Maiden, M.; Meyer, T. F.; Montecucco, C.; Oswald, E.; Parkhill, J.; Pucciarelli, M. G.; Ron, E.; Svanborg, C.; Uhlin, B. E.; et al. (2008). "Pathogenomics: An updated European Research Agenda". Infection, Genetics and Evolution. 8 (3): 386–93. doi:10.1016/j.meegid.2008.01.005. PMID 18321793.
- ↑ Fleischmann, R.D.; Adams, M.D.; White, O.; Clayton, R.A.; Kirkness, E.F.; Kerlavage, A.R.; Bult, C.J.; Tomb, J.F.; Dougherty, D.A.; Merrick, J.M.; et al. (1995). "Whole-genome random sequencing and assembly of Haemophilus influenzae". Science. 269 (5223): 496–512. doi:10.1126/science.7542800. PMID 7542800.
- 1 2 3 4 5 6 7 8 9 10 11 Pallen M.J & Wren B.W. (2007). "Bacterial Pathogenomics". Nature. 449 (7164): 835–842. doi:10.1038/nature06248. PMID 17943120.
- ↑ Hall, N. (2007). "Advanced sequencing technologies and their wider impact in microbiology". J. Exp. 210 (Pt 9): 1518–1525. doi:10.1242/jeb.001370. PMID 17449817.
- 1 2 3 4 5 6 7 8 9 10 11 12 Fraser-Liggett, C. M. (2005). "Insights on biology and evolution from microbial genome sequencing". Genome Research. 15 (12): 1603–10. doi:10.1101/gr.3724205. PMID 16339357.
- ↑ Rogers, Y.-H.; Venter, J.C. (2005). "Genomics: Massively parallel sequencing". Nature. 437 (7057): 326–327. doi:10.1038/437326a. PMID 16163333.
- ↑ Raskin, David M.; Seshadri, Rekha; Pukatzki, Stefan U.; Mekalanos, John J. (2006). "Bacteria genomics and pathogen evolution". Cell. 124 (4): 703–714. doi:10.1016/j.cell.2006.02.002. PMID 16497582.
- 1 2 3 Perna, Nicole T.; Plunkett, III, Guy; Burland, Valerie; Mau, Bob; Glasner, Jeremy D.; Rose, Debra J.; Mayhew, George F.; Evans, Peter S.; Gregor, Jason; Kirkpatrick, Heather A.; et al. (2001). "Genome sequence of enterohaemorrhagic Escherichia coli O157:H7". Nature. 409 (6819): 529–533. doi:10.1038/35054089. PMID 11206551.
- ↑ Rappuoli, R. (2001). "Reverse Vaccinology". Vaccine. 19 (17–19): 2688–2691. doi:10.1016/S0264-410X(00)00554-5. PMID 11257410.
- ↑ Ward, P. N.; Holden, M. T.; Leigh, J. A.; Lennard, N.; Bignell, A.; Barron, A.; Clark, L.; Quail, M. A.; Woodward, J.; Barrell, B. G.; Egan, S. A.; Field, T. R.; Maskell, D.; Kehoe, M.; Dowson, C. G.; Chanter, N.; Whatmore, A. M.; Bentley, S. D.; Parkhill, J. (2009). "Evidence for niche adaptation in the genome of the bovine pathogen Streptococcus uberis". BMC Genomics. 10: 54. doi:10.1186/1471-2164-10-54. PMC 2657157. PMID 19175920.
- ↑ Cole, S.T., Eiglmeierr, K., Parkhill, J., James, K.D., Wheeler, P.R., Honore, N., Garnier, T., Churcher, C., Harris, D., Mungall, K; Eiglmeier; Parkhill; James; Thomson; Wheeler; Honoré; Garnier; Churcher; Harris; Mungall; Basham; Brown; Chillingworth; Connor; Davies; Devlin; Duthoy; Feltwell; Fraser; et al. (2001). "Massive gene decay in the leprosy bacillus". Nature. 409 (6823): 1007–11. doi:10.1038/35059006. PMID 11234002.
- 1 2 Parkhill, J.; Wren, B. W.; Thomson, N. R.; Titball, R. W.; Holden, M. T. G.; Prentice, M. B.; Sebaihia, M.; James, K. D.; Churcher, C.; Mungall, K. L.; Baker, S.; Basham, D.; Bentley, S. D.; Brooks, K.; Cerdeño-Tárraga, A. M.; Chillingworth, T.; Cronin, A.; Davies, R. M.; Davis, P.; Dougan, G.; Feltwell, T.; Hamlin, N.; Holroyd, S.; Jagels, K.; Karlyshev, A. V.; Leather, S.; Moule, S.; Oyston, P. C. F.; Quail, M.; et al. (2001). "Genome sequence of Yersinia pestis, the causative agent of plague". Nature. 413 (6855): 523–7. doi:10.1038/35097083. PMID 11586360.
- ↑ Parkhill J, Dougan G, James KD, Thomson NR, Pickard D, Wain J, Churcher C, Mungall KL, Bentley SD, Holden MT, Sebaihia M, et al. (2001). "Complete genome sequence of a multiple drug resistant Salmonella enterica serovar Typhi CT18". Nature. 413 (6858): 848–852. doi:10.1038/35101607. PMID 11677608.
- 1 2 3 Hain T, Chatterjee SS, Ghai R, Kuenne CT, Billion A, Steinweg C, Domann E, Kärst U, Jänsch L, Wehland J, Eisenreich W, Bacher A, et al. (2007). "Pathogenomics of Listeria spp". Int J Med Microbiol. 297 (7–8): 541–57. doi:10.1016/j.ijmm.2007.03.016. PMID 17482873.
- ↑ Boucher Y, Douady CJ, Papke RT, Walsh DA, Boudreau ME, Nesbø CL, Case RJ, Doolittle WF. (2003). "Lateral gene transfer and the origins of prokaryotic groups". Annu Rev Genet. 37: 283–328. doi:10.1146/annurev.genet.37.050503.084247. PMID 14616063.
- ↑ Lima WC, Paquola AC, Varani AM, Van Sluys MA, Menck CF. (2008). "Laterally transferred genomic islands in Xanthomonadales related to pathogenicity and primary metabolism". FEMS Microbiol. Lett. 281 (1): 87–97. doi:10.1111/j.1574-6968.2008.01083.x. PMID 18318843.
- ↑ Gill SR, Fouts DE, Archer GL, Mongodin EF, Deboy RT, Ravel J, Paulsen IT, Kolonay JF, Brinkac L, Beanan M, Dodson RJ, Daugherty SC, Madupu R, Angiuoli SV, et al. (2005). "Insights on Evolution of Virulence and Resistance from the Complete Genome Analysis of an Early Methicillin-Resistant Staphylococcus aureus Strain and a Biofilm-Producing Methicillin-Resistant Staphylococcus epidermidis Strain". J Bacteriol. 187 (7): 2426–38. doi:10.1128/JB.187.7.2426-2438.2005. PMC 1065214. PMID 15774886.
- ↑ Bapteste E, Boucher Y. (2008). "Lateral gene transfer challenges principles of microbial systematics". Trends Microbiol. 16 (5): 200–7. doi:10.1016/j.tim.2008.02.005. PMID 18420414.
- ↑ Huang J, Gogarten JP. (2006). "Ancient horizontal gene transfer can benefit phylogenetic reconstruction". Trends Genet. 22 (7): 361–6. doi:10.1016/j.tig.2006.05.004. PMID 16730850.
- ↑ H. and Moran; Mira, A., Ochman, N. A.; Moran, NA (2001). "Deletional bias and the evolution of bacterial genomes". Trends Genet. 17 (10): 589–596. doi:10.1016/S0168-9525(01)02447-7. PMID 11585665.
- ↑ Nierman WC, DeShazer D, Kim HS, Tettelin H, Nelson KE, Feldblyum T, Ulrich RL, Ronning CM, Brinkac LM, Daugherty SC, et al. (2004). "Structural flexibility in the Burkholderia mallei genome". Proc Natl Acad Sci USA. 101 (39): 14246–51. doi:10.1073/pnas.0403306101. PMC 521142. PMID 15377793.
- ↑ Holden MT, Titball RW, Peacock SJ, Cerdeño-Tárraga AM, Atkins T, Crossman LC, Pitt T, Churcher C, Mungall K, Bentley SD, Sebaihia M, Thomson NR, Bason N, Beacham IR, Brooks K, Brown KA, et al. (2001). "Genomic plasticity of the causative agent of melioidosis, Burkholderia pseudomallei". Proc Natl Acad Sci USA. 101 (39): 14240–5. doi:10.1073/pnas.0403302101. PMC 521101. PMID 15377794.
- 1 2 3 4 Tettelin H, Masignani V, Cieslewicz MJ, Donati C, Medini D, Ward NL, Angiuoli SV, Crabtree J, Jones AL, Durkin AS, Deboy RT, Davidsen TM, Mora M, Scarselli M, Margarit y Ros I, Peterson JD, Hauser CR, et al. (2005). "Genome analysis of multiple pathogenic isolates of Streptococcus agalactiae: Implications for the microbial "pan-genome"". Proc Natl Acad Sci USA. 102 (39): 13950–5. doi:10.1073/pnas.0506758102. PMC 1216834. PMID 16172379.
- ↑ Lapierre P, Gogarten JP. (2009). "Estimating the size of the bacterial pan-genome". Trends Genet. 25 (3): 107–10. doi:10.1016/j.tig.2008.12.004. PMID 19168257.
- 1 2 3 Medini D, Donati C, Tettelin H, Masignani V, Rappuoli R. (2005). "The microbial pan-genome". Current Opinion in Genetics & Development. 15 (6): 589–94. doi:10.1016/j.gde.2005.09.006. PMID 16185861.
- ↑ Tettelin H, Riley D, Cattuto C, Medini D. (2008). "Comparative genomics: the bacterial pan-genome". Current Opinion in Microbiology. 11 (5): 472–7. doi:10.1016/j.mib.2008.09.006. PMID 19086349.
- ↑ Langille MG, Brinkman FS. (2009). "IslandViewer: an integrated interface for computational identification and visualization of genomic islands". Bioinformatics. 25 (5): 664–5. doi:10.1093/bioinformatics/btp030. PMC 2647836. PMID 19151094.
- ↑ Guy L.. (2006). "Identification and characterization of pathogenicity and other genomic islands using base composition analyses". Future Microbiol. 1 (3): 309–16. doi:10.2217/17460913.1.3.309. PMID 17661643.
- ↑ "Research on microbial biofilms (PA-03-047)". NIH, National Heart, Lung, and Blood Institute. 2002-12-20.
- ↑ Valle J, Vergara-Irigaray M, Merino N, Penadés JR, Lasa I. (2007). "σB Regulates IS256-Mediated Staphylococcus aureus Biofilm Phenotypic Variation". J Bacteriol. 189 (7): 2886–96. doi:10.1128/JB.01767-06. PMC 1855799. PMID 17277051.
- ↑ Hogardt M, Hoboth C, Schmoldt S, Henke C, Bader L, Heesemann J. (2007). "Stage-specific adaptation of hypermutable Pseudomonas aeruginosa isolates during chronic pulmonary infection in patients with cystic fibrosis". J Infect Dis. 195 (1): 70–80. doi:10.1086/509821. PMID 17152010.
- 1 2 Cheng LW, Viala JP, Stuurman N, Wiedemann U, Vale RD, Portnoy DA. (2005). "Use of RNA interference in Drosophila S2 cells to identify host pathways controlling compartmentalization of an intracellular pathogen". Proc Natl Acad Sci U S A. 102 (38): 13646–51. doi:10.1073/pnas.0506461102. PMC 1224656. PMID 16157870.
- ↑ Hattori M, Taylor TD. (2009). "The Human Intestinal Microbiome: A New Frontier of Human Biology". DNA Res. 16 (1): 1–12. doi:10.1093/dnares/dsn033. PMC 2646358. PMID 19147530.
- ↑ Hooper LV, Gordon JI. (2001). "Commensal host-bacterial relationships in the gut". Science. 292 (5519): 1115–8. doi:10.1126/science.1058709. PMID 11352068.
- ↑ Oshima K, Toh H, Ogura Y, Sasamoto H, Morita H, Park S-H, Ooka T, Iyoda S, Taylor TD, Hayashi T, Itoh K, Hattori M. (2008). "Complete Genome Sequence and Comparative Analysis of the Wild-type Commensal Escherichia coli Strain SE11 Isolated from a Healthy Adult". DNA Res. 15 (6): 375–86. doi:10.1093/dnares/dsn026. PMC 2608844. PMID 18931093.
- ↑ Zoetendal EG, Rajilic-Stojanovic M, de Vos WM (2008). "High-throughput diversity and functionality analysis of the gastrointestinal tract microbiota". Gut. 57 (11): 1605–15. doi:10.1136/gut.2007.133603. PMID 18941009.
- ↑ Achtman M, Morelli G, Zhu P, Wirth T, Diehl I, Kusecek B, Vogler AJ, Wagner DM, Allender CJ, Easterday WR, Chenal-Francisque V, Worsham P, Thomson NR, Parkhill J, Lindler LE, Carniel E, Keim P (2004). "Microevolution and history of the plague bacillus, Yersinia pestis". Proc. Natl. Acad. Sci. USA. 101 (51): 17837–17842. doi:10.1073/pnas.0408026101. PMC 535704. PMID 15598742.
- 1 2 Serruto D, Serino L, Masignani V, Pizza M. (2009). "Genome-based approaches to develop vaccines against bacterial pathogens". Vaccine. 27 (25–26): 3245–50. doi:10.1016/j.vaccine.2009.01.072. PMID 19200820.
- 1 2 Muzzi A, Masignani V, Rappuoli R. (2007). "The pan-genome: towards a knowledge-based discovery of novel targets for vaccines and antibacterials". Drug Discov Today. 12 (11–12): 429–39. doi:10.1016/j.drudis.2007.04.008. PMID 17532526.
- ↑ Taubenberger JK, Reid AH, Lourens RM, Wang R, Jin G, Fanning TG. (2005). "Characterization of the 1918 influenza virus polymerase genes". Nature. 437 (7060): 889–93. doi:10.1038/nature04230. PMID 16208372.
- ↑ Tumpey TM, Basler CF, Aguilar PV, et al. (October 2005). "Characterization of the reconstructed 1918 Spanish influenza pandemic virus". Science. 310 (5745): 77–80. doi:10.1126/science.1119392. PMID 16210530.
- 1 2 Kaiser J (2005). "Resurrected influenza virus yields secrets of deadly 1918 pandemic". Science. 310 (5745): 28–9. doi:10.1126/science.310.5745.28. PMID 16210501.
- ↑ "Terrorism Project)". Center for Defense Information. 2002-12-20.
- ↑ van Aken J. (2007). "Ethics of reconstructing Spanish flu: is it wise to resurrect a deadly virus?". Heredity. 98 (1): 1–2. doi:10.1038/sj.hdy.6800911. PMID 17035950.